A coronavirus vaccine or even several could be ready in a few months, so experts are beginning to worry about how to get it into people's arms.
“Vaccines don’t save lives. Vaccinations save lives,” said Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins Bloomberg School of Public Health.
USA TODAY's expert panelists, increasingly optimistic about the prospect of a readily available vaccine, are concerned about who will get it first, how doses will be shipped and what messages the government must send so Americans trust getting one.
As the prospect of an immunization for COVID-19 comes into focus, educating the public about how vaccines will work and the painstaking process required to approve them will be key to getting people to line up once they’re available.
It will take “messaging the integrity of the research system,” said Sam Halabi, a law professor at the University of Missouri and an expert on global health law.
If this doesn’t happen now, the more than $10 billion in tax money spent on vaccines will have been wasted because not enough people will get them to matter.
The agencies that decide whether a vaccine is ready and who gets it first – the Food and Drug Administration and the U.S. Centers for Disease Control and Prevention – need to be front and center and as transparent as possible, said Dr. William Schaffner, a professor and infectious disease expert at Vanderbilt University School of Medicine in Nashville, Tennessee.
“It is vital that the U.S. government communicate clearly with the American people about the data and science behind any new COVID-19 vaccine,” said Dr. Michelle McMurry-Heath, president and CEO of the Biotechnology Innovation Organization.
A few public officials getting immunizations wouldn’t hurt, said Pamela Bjorkman, a structural biologist at the California Institute of Technology.
“If/when there is a vaccine, government officials should be filmed getting the vaccine,” she said.
This has to happen at full throttle. A vaccine or vaccines could be ready within months, so time is of the essence, they said.
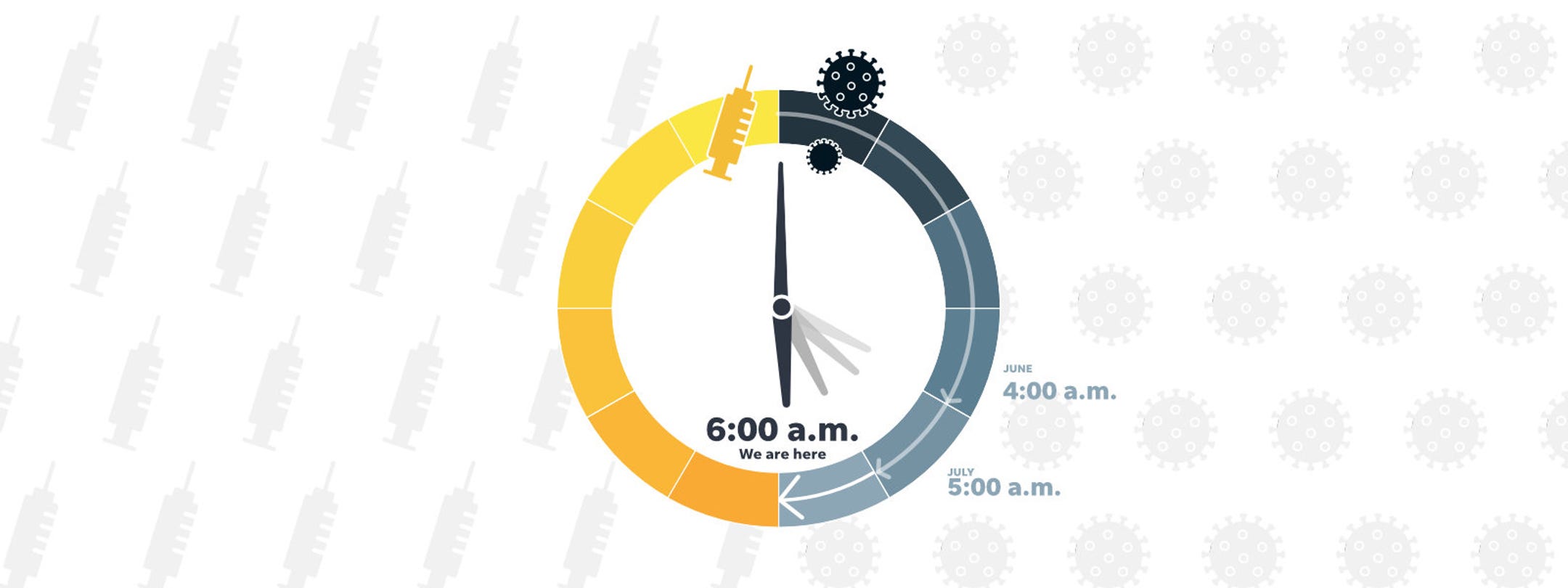
Dawn is breaking on a virus vaccine
To understand when pre-COVID-19 life in America can resume, USA TODAY created a panel of experts in medicine, virology, immunology, logistics and supply chain issues to estimate how close we are to securing a vaccine against SARS-CoV-2, the virus that causes COVID-19.
Every month, these experts estimate where they think things stand and what time it is on the USA TODAY vaccine clock. Midnight is the starting point of the pandemic in the USA, and noon is the time a vaccine will be widely available to Americans.
For August, the clock’s hands stand at 6 a.m., two hours closer to noon than in June, the first month of estimates. Dawn is breaking, but the security of a vaccine against the disease that has ravaged the world is still far away.
This month, the 15 estimates ranged from 4 a.m. to a highly optimistic 10 a.m. The median was 6 a.m.
Don’t put all vaccine eggs in one basket
It’s far too soon to presume victory, panelists said.
There’s still a possibility that none of the vaccine candidates will pan out. The typical success rate for vaccine development is 6%, according to The Lancet, a British medical journal.
“We simply cannot assume the outcome at this stage,” said Dr. Kelly Moore, associate director of immunization education with the Immunization Action Coalition.
“The government should hedge its bets and put more money into basic vaccine research. If these don’t work, then we’re going to be back to square one. Getting square one started now is important. And even if they do work, that that kind of research work on new vaccine platforms is not a waste,” said Dr. Otto Yang, a professor of medicine and chief of infectious disease at the David Geffen School of Medicine at UCLA.
This may not be one-and-done either, said Dr. Greg Poland, director of the Mayo Clinic's Vaccine Research Group.
Just as the common cold and flu mutate a little each year, coronavirus could do the same. Funding new vaccine development that can fill the void in case of viral mutation/recombination will be critical, said Poland, editor-in-chief of the journal Vaccine.
It’s the homestretch but a long one
At least 10 possible vaccines are in the final Phase 3 clinical testing stage. Will they work, and will they work long enough?
There's no data to show that any of the candidate vaccines provides immunity to COVID-19. That’s what Phase 3 clinical trials test for, and they are just starting.
“This is a new virus, and we are just beginning to unravel the protective immunity,” said Prakash Nagarkatti, an immunologist and vice president for research at the University of South Carolina.

Phase 1 testing is done on small numbers of volunteers to ensure the candidate vaccine is safe and doesn’t have common, serious side effects. Phase 2 increases to a few hundred volunteers and sets a safe dose. It isn’t until Phase 3, set at 30,000 volunteers by the National Institutes of Health, that data shows whether it protects people against COVID-19.
Dr. Monica Gandhi, an infectious disease expert at the University of California-San Francisco, said data from people who’ve recovered from COVID-19 makes it seem likely that immunity is possible.
“Natural infectious always gives you a clue to immunity, and I think natural infection with this virus seems to be giving people immunity,” she said.
Even if immunity lasts only a year, booster immunizations can be given if vaccine efficacy wanes over time, Bjorkman said. The only way to know for certain will be to evaluate data from Phase 3 trials, she said.
Even so, simply having a vaccine that worked for a year at a time would turn COVID-19 from a pandemic into something manageable, just like the seasonal flu, said Erica Ollmann Saphire, a structural biologist and professor at the La Jolla Institute for Immunology.
“We’re willing to take a seasonal coronavirus vaccine if that’s what it takes until something universal and lifelong is developed,” she said.
Securing a vaccine not a slam dunk
The public needs to remember that life isn’t going to suddenly zip back to the way it was in January, when we all lived blissfully unaware of the virus or its effects, the experts said.
It’s not likely any vaccine will be 100% effective, and it could be that people who are immunized might still get sick but less severely.
“I think we’d have the chance of having a vaccine that substantially blunts the frequency of severe disease and therefore reduces mortality,” Schaffner said.
That alone will be a huge win, but the need to keep wearing masks and socially distance might still be there.
“I don’t think we’ve communicated that,” he said.
This month's panelists
Pamela Bjorkman, structural biologist at the California Institute of Technology
Dr. Monica Gandhi, an infectious disease expert at the University of California, San Francisco
Sam Halabi, professor of law, University of Missouri; scholar at the O’Neill Institute for National and Global Health Law at Georgetown University.
Florian Krammer, virologist at the Icahn School of Medicine at Mount Sinai in New York City
Dr. Michelle McMurry-Heath, president and CEO of Biotechnology Innovation Organization
Dr. Kelly Moore, associate director of immunization education, Immunization Action Coalition; former member of the CDC Advisory Committee on Immunization Practices; chair, World Health Organization Immunization Practices Advisory Committee
Prakash Nagarkatti, immunologist and vice president for research, University of South Carolina
Peter Pitts, president of the Center for Medicine in the Public Interest,
Dr. Gregory Poland, director, Mayo Clinic's Vaccine Research Group, editor-in-chief, Vaccine
Arti Rai, law professor and health law expert at Duke University Law School
Daniel Salmon, director of the Institute for Vaccine Safety at Johns Hopkins Bloomberg School of Public Health
Erica Ollmann Saphire, structural biologist and professor at La Jolla Institute for Immunology
Dr. William Schaffner, professor of preventive medicine, Department of Health Policy, and professor of medicine, Division of Infectious Diseases, Vanderbilt University
Prashant Yadav, senior fellow, Center for Global Development, medical supply chain expert
Dr. Otto Yang, a professor of medicine and chief of infectious disease at the David Geffen School of Medicine at UCLA
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.