PCSK9 Inhibitors: What You Need to Know
Steph’s Note: Today we introduce a new guest author to the site. Jessica Boh, PharmD, BCPS is a current PGY2 Pharmacy Resident in Ambulatory Care. She is also completing a Masters in Public Health (MPH). She hopes to stay within the VA system as an Ambulatory Care pharmacist, where she loves serving Veterans and the challenge of practicing at the top of her license. Also after residency, Jessica intends to reconnect with her favorite pastime - belly dance - which she has been studying, performing, and teaching for over 10 years!
Lower my LDL-ah ella ella, eh eh eh!! (Image)
If you ever want to sound smart in a crowded room, start talking about PCSK9 inhibitors.
These hot and relatively new medications are gaining indications and popularity left-and-right for LDL-lowering benefit (that’s the bad cholesterol) and prevention of adverse cardiovascular events.
So, what’s the skinny on these lipid-lowering agents? I’m about to break it down like hepatocytes.
And BTW, to help you figure out where PCSK-9 inhibitors fit in the treatment landscape, check out our Hyperlipidemia Cheat Sheet. It’s packed with dosing info, drug interactions, clinical pearls, and all sorts of other goodies (including a breakdown of all EIGHT(?!) formulations of fenofibrate).
Why is PCSK9 a good drug target?
Pro tip from my girl Betsy to remember the order of PCSK9: sounds like “please say yes (to the) canine”. Because how could you not??? (Image)
Proprotein convertase subtilisin/kexin type 9 (man, that’s a mouthful) is an enzyme that is found mainly in the liver, but it is also found in the kidneys, intestines, and central nervous system. It binds to LDL receptors (LDL-R) on the hepatocyte surface, resulting in degradation of the LDL-R.
We rely on the LDL-R to grab LDL cholesterol (LDL-C) in the blood and pull it into the cell for metabolism. So high levels of PCSK9 = fewer functioning LDL-R = higher levels of LDL-C floating around, wreaking havoc.
Therefore, PCSK9 inhibitors lead to higher numbers of functioning LDL-R on hepatocytes and increased uptake of LDL-C into the hepatocyte, thus decreasing circulating LDL-C.
Pharmacology of PCSK9 Inhibitors
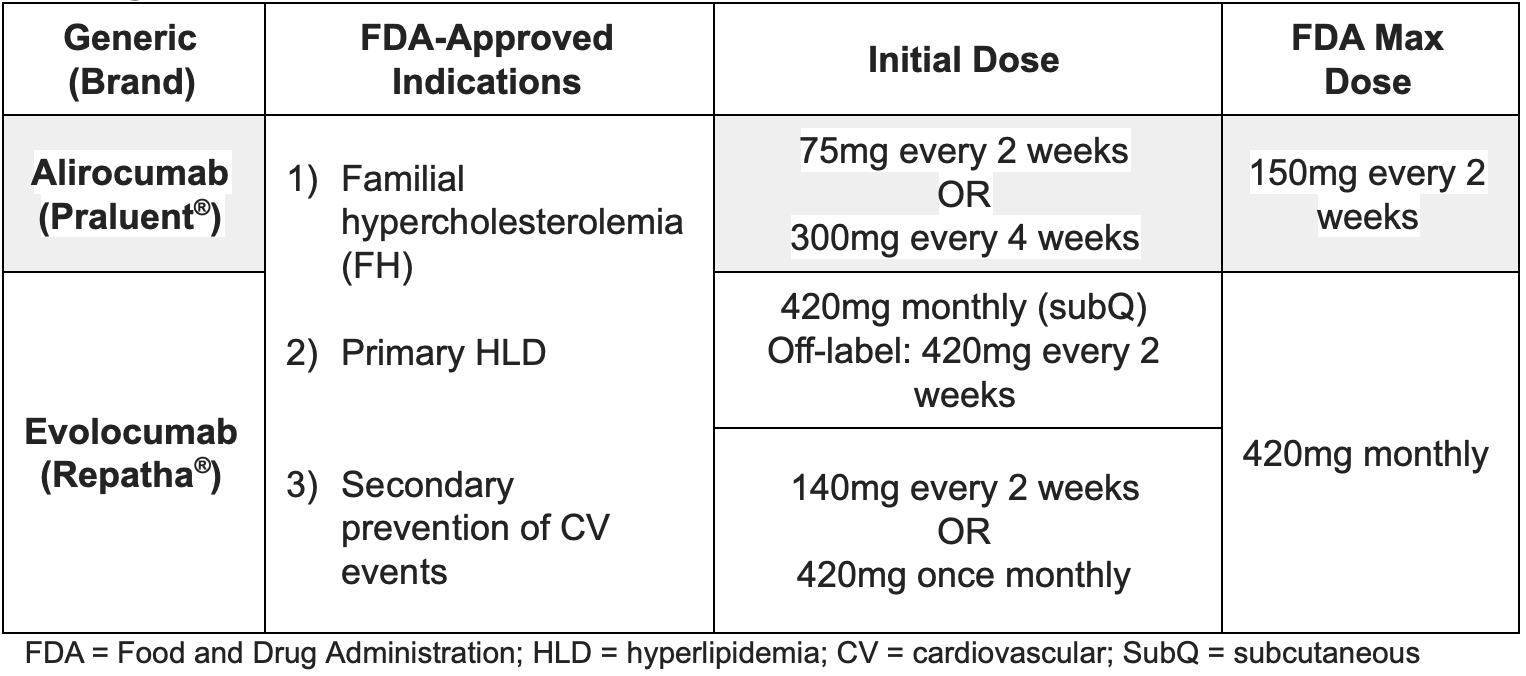
There are two PCSK9 inhibitors currently available in the US: alirocumab (Praluent) and evolocumab (Repatha). Both are monoclonal antibodies that bind to and inhibit the PCSK9 enzyme.
Alirocumab is an immunoglobulin G (IgG) 1 isotype, whereas evolocumab is an IgG2 isotype. Clinically, the isotype doesn’t mean much except:
They were able to get separate patents ($$$), and
If one for some reason doesn’t work or produces unwanted side effects, it may be reasonable to try the other.
Dosing of PCSK9 Inhibitors
How effective are PCSK9 Inhibitors?
Once upon a time when evolocumab was the only drug available in this class, it was mainly used to treat a genetic condition called familial hyperchol…hypercholester…too much cholesterol in the blood.
Familial hypercholesterolemia (FH) can be heterozygous or homozygous. People with FH can have a wide range of genetic mutations in the LDL-R gene, the PCSK9 enzyme gene, or the apolipoprotein B gene (APOB3500). These mutations can lead to crazy high LDL levels in the blood - so high they need a procedure called apheresis to remove it from the blood!
This type of medication worked so well in FH that people started to wonder… What about using them in other people with high LDL, but without FH?
Spoiler alert, it worked really well in those patients, too!
(Image)
How are PCSK9 inhibitors given?
Both alirocumab and evolocumab are self-administered subcutaneously. When counseling patients, be sure to remind them to take the medication out of the fridge to un-chill for 30-40 minutes before using (unless you really dislike them, ouch! Kidding…!). As with babies, resist the temptation to shake! Also, don’t try to speed up the heating process with hot water, direct sunlight, or the microwave…
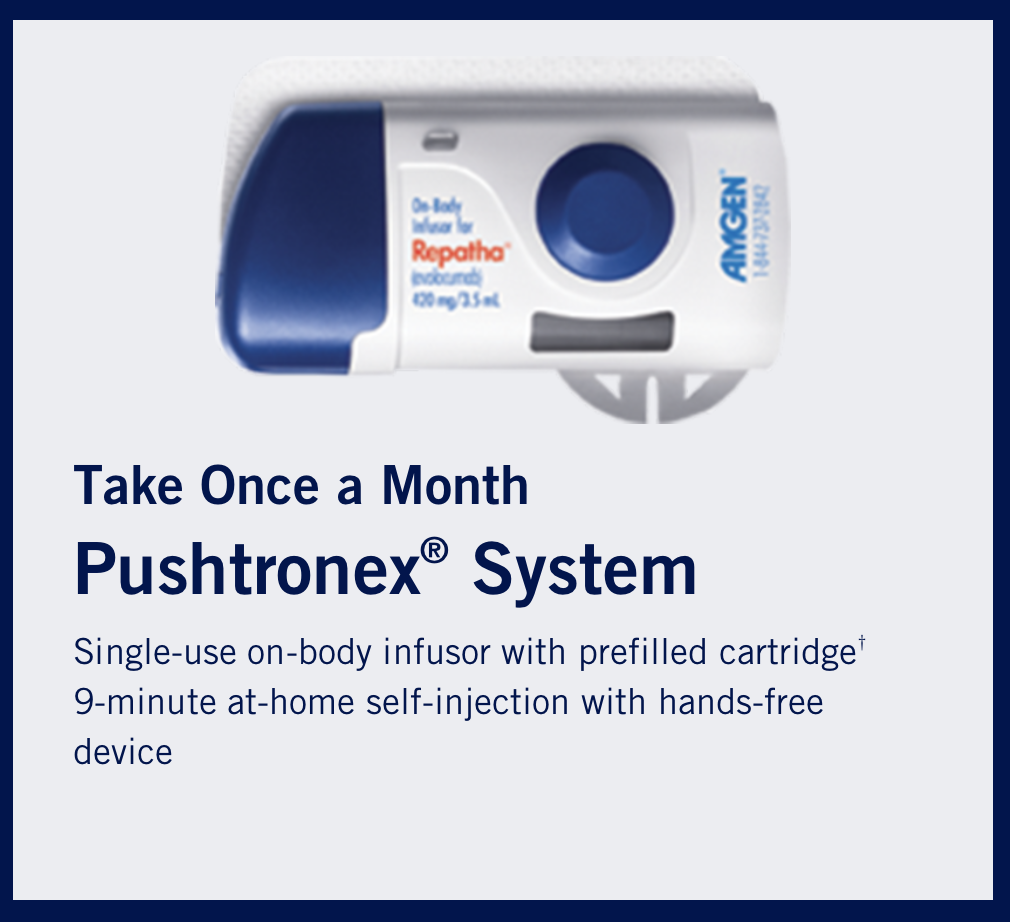
The fancy-pants evolcumab infusion device as an alternative to the pen and auto injector. (Image)
For the alirocumab 300 mg dose, patients should inject two 150 mg doses consecutively at separate sites. For the evolocumab 420 mg dose, the patient may inject three separate 140 mg injections at separate sites within a 30-minute period.
Um, can I get another ouch?? That’s borderline pin cushion!
To avoid feeling like that, some patients can get the fancy-pants evolocumab on-body infusion device, which delivers the 420 mg dose subcutaneously over 9 minutes.
If patients are getting apheresis, these agents can be administered regardless of timing with this procedure. Woot! One less medication to worry about being pulled off and eliminated!
Traveling tip for patients: the pens are only good out of the fridge for 30 days.
What side effects should we expect with PCSK9 inhibitors?
For the most part, both alirocumab and evolocumab are pretty well tolerated, which is more than we can say for statins (just reading the package insert makes me ache). The most commonly reported adverse drug reaction (ADR) with alirocumab is redness/itching at the injection site, and the most commonly reported ADR with evolocumab is nasopharyngitis, per the package inserts.
However, in the big ol’ trials, injection site reactions were the only ADRs with a statistically significantly increased risk compared with placebo, and these were still relatively rare (1-2%). Both PCSK9 inhibitors can cause rare but serious hypersensitivity reactions, so it’s important to counsel patients on the signs and symptoms of an allergic or hypersensitivity reaction.
Fun fact, the blockbuster PCSK9 inhibitor bococizumab that came out first is no longer a thing and the SPIRE trial was stopped early due to hypersensitivity reactions.
From the ODYSSEY OUTCOMES trial, antidrug antibodies were reported in 0.7% of participants on alirocumab versus 0.4% with placebo. It doesn’t appear so far that evolocumab or alirocumab have the same immunogenic effects. Other much less commonly reported ADRs include increased transaminases (alirocumab), influenza, cough, myalgia, diabetes (evolocumab), and hypertension (evolocumab).
How do we monitor PCSK9 inhibitors?
Labs
Of course, you’ll want to obtain a baseline lipid panel. This can be either fasting or non-fasting. Re-check fasting lipids 4-12 weeks after initiation of therapy and then every 3-12 months thereafter. You should expect to see a pretty dang good response by week 12.
LDL Targets
For a while there (ahem…the 2013 ACC/AHA Guidelines) we got away from worrying about LDL numbers and just thinking about appropriate statin intensity. Now that we have some other treatment options and more data about the relationship of LDL and CV events, new LDL goals have emerged.
The American Association of Clinical Endocrinologists and American College of Endocrinology (AACE) 2017 Guidelines suggest targeting LDL < 55 mg/dL in “extreme risk” patients. So who’s at extreme risk?
Patients with the following:
Events even with LDL of 70 mg/dL
Established atherosclerotic cardiovascular disease (ASCVD)
Diabetes mellitus (DM)
Chronic kidney disease (CKD) stages 3 or 4
Heterozygous FH
History of premature ASCVD
They suggest for “very high risk” patients to target LDL < 70 mg/dL, such as those with recent acute coronary syndromes (ACS) or 10-year risk > 20%, DM, or CKD 3/4 with 1 or more risk factors, or heterozygous FH.
Everyone else they say LDL < 100 mg/dL. Or if there are absolutely no risk factors at all, then LDL < 130 mg/dL.
How low is too low? Is there a too low when it comes to cholesterol?
It appears to be true that there is a unit-for-unit lower risk of CV events with every decrease in LDL (22% reduction in events per every 40 mg/dL decrease in LDL to be exact). In the FOURIER trial, subjects who achieved LDL < 10 mg/dL had a 40% lower risk of CV events compared to the subjects who achieved LDL of 100 mg/dL. So why not shoot for LDL < 10 mg/dL? Heck, why not just go all the way and shoot for an LDL of 0 mg/dL (which, yes, is definitely achievable with PCSK9 inhibitors in clinical practice - I’ve seen it!)?
(Image)
There are theoretical concerns with very low LDL. Cholesterol does play a role in normal cell function, so without it or with very low levels, do we create unhealthy cell membranes and neurocognitive defects?
What we do know is there are people walking around with genetic mutations in their PCSK9 enzyme that actually cause them to lose function of the enzyme, resulting in naturally low LDL levels around ~15 mg/dL. To date, there are no established adverse effects associated with this genetic mutation. In the FOURIER trial, the median LDL after treatment was 30 mg/dL, and in the IMPROVE-IT trial with ezetimibe + statin, it was 54 mg/dL, with low adverse drug event rates.
However, given the theoretical concerns and lack of long-term data, several targets such as LDL > 40 mg/dL have been suggested by expert opinion. The jury is still out on this one.
Show me the numbers for PCSK9 inhibitors!
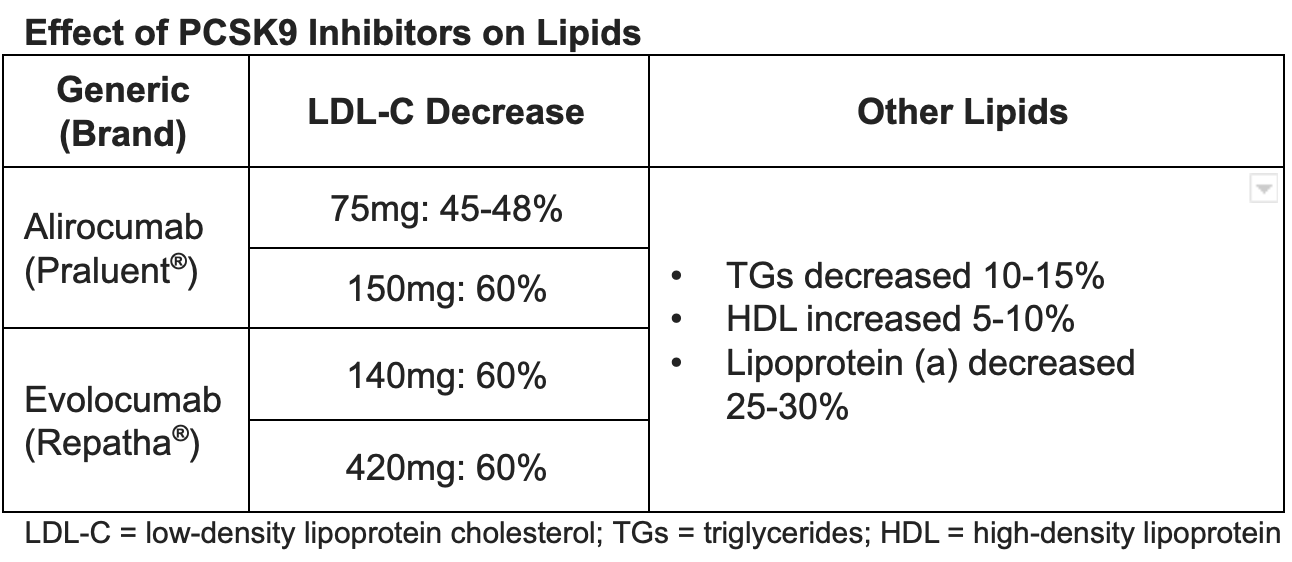
LDL-Lowering Benefits
A number of trials showed excellent lipid-lowering benefit in a variety of participant populations. The GAUSS-2 and LAPLACE-2 trials showed greater LDL reductions with evolocumab when compared with ezetimibe. GAUSS-2 included participants who were intolerant to 2 or more statins with an LDL still above goal. It resulted in an LDL reduction of 53-56% with evolocumab compared with 37-39% with ezetimibe. There was significantly more myalgia with ezetimibe compared with evolocumab.
LAPLACE-2 included patients with HLD and various strengths of statins, including patients who were statin intolerant. At 12 weeks, evolocumab reduced LDL up to 75%. Other trials including DESCARTES, YUKAWA, RUTHERFORD-2, and MENDEL-2 also showed excellent LDL reduction with evolocumab.
Pleiotropic Effects?
Remember that NAPLEX word “pleiotropic” effects that we use to describe statins?
Pleiotropic effects are those things outside of the LDL-lowering benefit that we know statins can do, such as stabilizing plaques, altering endothelial nitric oxide synthase expression, reduce cardiac hypertrophy and fibrosis, and reducing platelet reactivity. But what about PCSK9 inhibitors?
Researchers are still finding out about how these medications really work, but it has been theorized that PCSK9 also plays a role in immune function and may be a target for sepsis (whaaat?). It may also be involved in plaque vulnerability, and alterations to it may modify the course of ACS, based on the fact that we know PCSK9 enzyme levels are elevated during myocardial ischemia.
So the PCSK9 inhibitors aren’t all about those numbers either. Cool.
Cardiovascular Outcomes
Ok so they work well to lower LDL, but what does this mean clinically? What’s the bottom line? Do the PCSK9 inhibitors prevent cardiovascular death?
The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial in 2017 included 27,564 subjects with established cardiovascular disease, LDL of at least 70 mg/dL, and who were on at least the statin equivalent of atorvastatin 20mg with or without ezetimibe. Subjects were randomized to receive either evolocumab or placebo. The primary endpoint was a composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization.
Evolocumab met its primary endpoint; however, the tricky thing about composite endpoints is that the individual components of that composite may or may not have show statistical significance. In the FOURIER trial, the endpoint was driven by myocardial infarction, with 3.4% in the evolocumab group vs 4.6% in placebo group (p < 0.001), and coronary revascularization, with 5.5% in the evolocumab group vs 7.0% in the placebo group (p < 0.001). The endpoint of cardiovascular death was not statistically significant, with 1.8% occurring in the evolocumab group and 1.7% in the placebo group.
The Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment with Alirocumab (ODYSSEY OUTCOMES) trial in 2018 included 18,924 participants with ACS within the past year and LDL at least 70 mg/dL OR non-HDL cholesterol at least 100 mg/dL OR apoB at least 80mg/dL. Subjects had to be on a maximally tolerated dose of statin, preferably high-intensity, and they were randomized to receive alirocumab or placebo.
Get this, they actually targeted an LDL range of 25-50 mg/dL. The primary endpoint was a composite of death from coronary heart disease (CHD), nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization.
Again, while alirocumab achieved statistical significance for reduction of any CHD event, any CV event, and the overall composite, it did not achieve statistical significance for reduction of death from CHD (2.2% in the alirocumab group versus 2.3% in the placebo group).
In both trials, strangely enough, the use of ezetimibe was relatively low with approximately 5% of patients in FOURIER and 3% in ODYSSEY OUTCOMES. The reasons are unclear. It is important to remember that ezetimibe does have evidence for reducing cardiovascular outcomes, as evidenced by the IMPROVE-IT trial.
In addition, it’s possible the trial follow-up just wasn’t long enough. The median follow-up for FOURIER was 2.2 years and 2.8 years for ODYSSEY OUTCOMES, compared with the statin-related mortality trials’ average of about 5 years follow-up.
What’s the takeaway on PCSK9 inhibitors?
PCSK9 inhibitors are super effective and generally well tolerated, but they’re also uber expensive. While there is evidence that these drugs decrease cardiovascular events such as heart attack and stroke, unlike our friends the statins, there is no evidence (yet) that they decrease mortality.
Therefore, it is important to continue using statins and ezetimibe as the backbones of lipid-lowering therapy and to consider adding a PCSK9 inhibitor when indicated if patients are intolerant to statins or their LDL is still not at goal on statins and ezetimibe.
Finally, there is a lack of evidence establishing the long-term effects of very low LDL; however, a number of minimum “soft target” LDLs have been suggested by expert opinion in the guidelines.
Hyperlipidemia Cheat Sheet
To figure out where PCSK-9 inhibitors fit in the treatment landscape, check out our Hyperlipidemia Cheat Sheet. It’s packed with dosing info, drug interactions, clinical pearls, and all sorts of other goodies (including a breakdown of all EIGHT(?!) formulations of fenofibrate).