Diabetes Management Part 1: Everything Insulin
Steph’s Note: J. Nile Barnes, PharmD, BCPS is back! Following his previous tl;dr series on heart failure and nephrology, this paramedic turned clinical pharmacist now working at the University of the Incarnate Word Feik School of Pharmacy in San Antonio, Texas is here again - this time to share his wealth of knowledge about insulin. If you haven’t read his other posts yet, #1, please do. Time seriously well spent. #2, you’ll quickly realize that this man is truly a teacher - his fascinating pharmacy history lessons trickle in vital information like pharmacokinetics such that they just stick like glue.
Also interspersed throughout this post is gotta-know info from Michelle Ton (4th year student at the UNC Eshelman School of Pharmacy in Chapel Hill, North Carolina), Sabrina Dunham (PGY1 at Moses Cone in Greensboro, North Carolina), Daniel Wadsworth and Taylor Sprague (3rd year students at UNC), and Jennifer Kim (clinical pharmacist faculty at Moses Cone and UNC). Talk about teamwork, right? Although we’re just briefly introducing this crew this week, stay tuned for Part 2 of this series since they’ll tackle regimen building strategies for diabetes in a follow up post next time.
Would you like to print this article? Or save it for offline viewing? You can get it as an attractive and printer-friendly PDF right here.
Insulin is the centerpiece of pharmacologic treatment for type 1 diabetes mellitus (T1DM) and has efficacy in managing type 2 diabetes mellitus (T2DM).
Insulin, an unsung hero in our daily lives, is a naturally-occurring hormone that is made by the pancreas. It allows our bodies to use sugar (glucose) from the food we eat as energy, or it stores the sugar for future use by modulating metabolism throughout the body, such as in the liver and various muscles. By doing so, it helps keep our blood sugars from getting too high (hyperglycemia) or too low (hypoglycemia). If a patient’s blood glucose is uncontrolled, they’re at risk for developing serious complications, like vascular damage or diabetic ketoacidosis (DKA), yikes!
Despite these benefits, insulin is also associated with quite a bit of frustration from both patients and pharmacists due to injections, fear of hypoglycemia, and cost. Fear not! Hopefully this article will help bring the important parts of insulin to your attention.
A Quick Review of Diabetes
The most recent Centers for Disease Control (CDC) statistic states that an estimated 23.1 million people in the US live with diabetes. Of this number, 5% have T1DM.
T1DM is typically diagnosed in children or young adults. As mentioned earlier, insulin is the only FDA-approved monotherapy for T1DM. Why’s that? Patients living with T1DM are unable to make their own insulin from their pancreas. Supplementing their daily insulins is essential to keep them alive (they are insulin-dependent). This is a disease state where lifestyle modifications aren’t going to help as much as the medicine (heellllooooo pharmacist intervention!).
But what about the rest of those patients living with T2DM? In T2DM, patients are considered insulin-resistant. Their pancreas has no problem producing insulin, but it’s either not enough or the liver is not responding to the insulin the way it should. Insulin is still used in T2DM, but depending on the severity, patients may be able to slide by with just metformin +/- other non-insulin agents (check out this awesome article about oral anti-diabetic medications in T2DM).
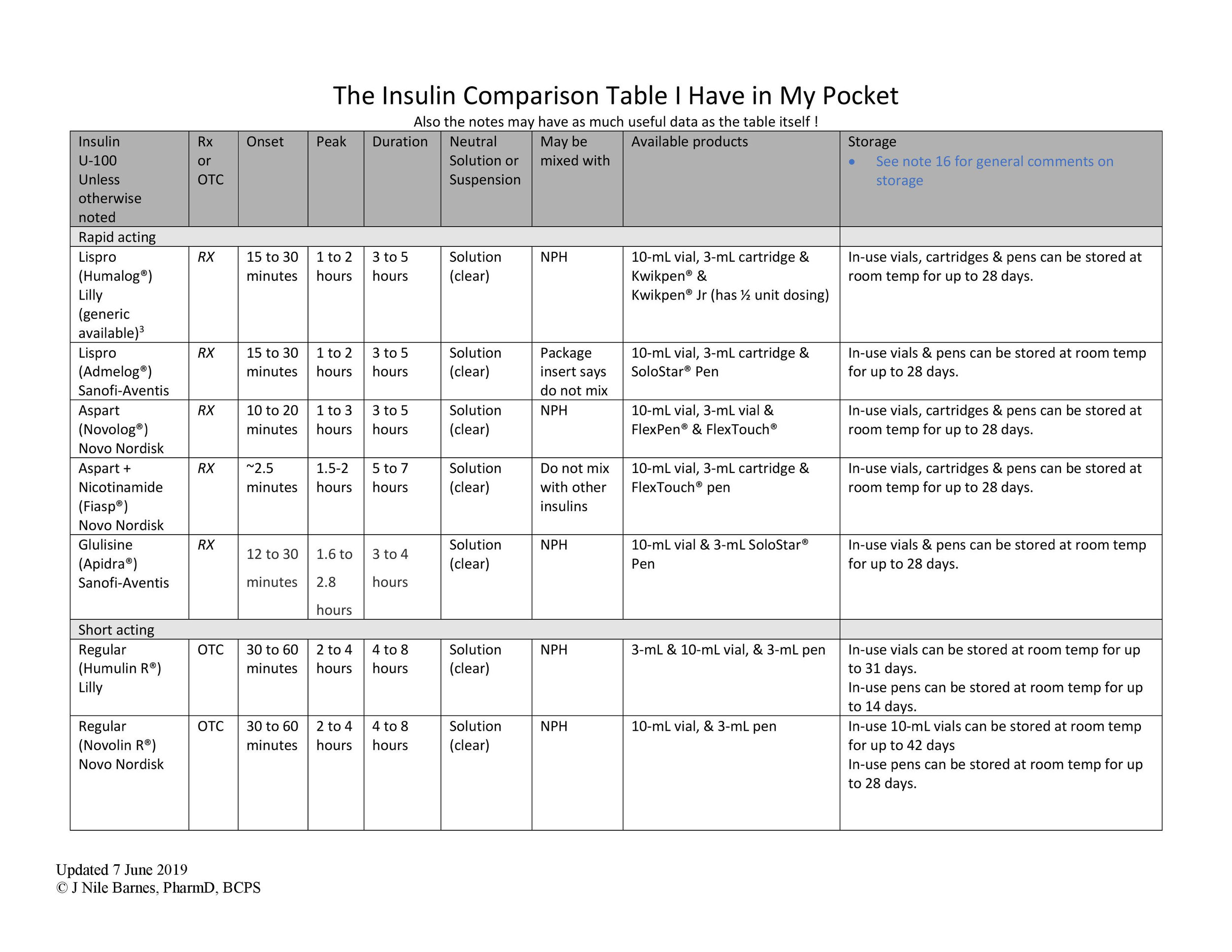
So, it’s time to start insulin on a patient. But…which one? (There are SO MANY as you can see by this amazing insulin table - courtesy of Dr. Barnes!)
Insulin R
Insulin N
Aspart (Which one, with the additive or not?)
Lispro (Which strength? Which one?)
Glulisine
Glargine (Which one? Which strength?)
Detemir
Degludec (Which strength?)
70/30
75/25
50/50
Or combination? Do you know the Brand® name? Is the brand important?
Does your patient have T1DM or T2DM? Does it matter?
What are the PK properties of the insulin you have chosen? Will it meet your patient’s needs?
To answer these questions and more, let’s start with some history. We know this is tl;dr, but it is important to have a little base knowledge here.
A Quick Trip Down Insulin Memory Lane
Banting and Best isolated insulin from dog Islets of Langerhans in the early 1920s at the University of Toronto in Professor JJR MacLeod’s laboratory. And after some initial problems were solved, Eli Lilly and company began to market extracted animal insulin for human use. Over the next 50-70 years, porcine and bovine insulins were available.
Diabetes mellitus could now be managed.
It is important to note that these were not prescription medications, although most patients did receive them via prescriptions. (By the way, they sold the patent to U of Toronto for $1. And Banting and MacLeod got a Nobel Prize for this in 1923, which they shared with their colleagues. Another fun fact, nearly 100 years after the initial discovery, the insulin molecule has gone on to reap at least two more Nobel Prizes - that’s kind of a big deal!)
Of course, this was porcine and bovine “Regular” insulin, which meant its pharmacokinetic profile mirrored (not exactly matched since these were animal proteins, not human) the insulin produced by the pancreas and therefore required multiple daily injections. It is also really important to know that exogenously administered insulin has a different PK profile than endogenously released insulin.
Trivia: these bovine and porcine products were not labelled as Regular at first…because there was nothing else to compare with.
When insulin is released endogenously, the effect lasts about five minutes; on the other hand, exogenous subcutaneous injection had an onset in about 30 minutes and had a few hours of duration. Why the difference?
(Image)
When exogenous insulin is injected in subcutaneous tissue, it aggregates into insoluble unavailable hexamers, which slowly degrade into dimers then monomers that are soluble. This Regular insulin was good for blunting prandial blood glucose peaks but did not last a very long time.
So “Regular” insulin is not perfect. To provide a longer duration of effect, insulins were mixed with various agents to slow absorption. The mechanisms used to slow absorption all stabilize the hexamers. The first products that made commercial success were insulin lente and insulin NPH. These contained varying amounts of zinc and protamine.
What’s this Insulin NPH?
Insulin NPH is still in widespread use. A little more about it…
In 1946, Hans Christian Hagedorn and colleagues introduced neutral crystalline protamine zinc insulin, also called isophane or Neutral Protamine (in the method of) Hagedorn (NPH) insulin.
This was the first real commercial success of an intermediate-duration insulin. Its effect lasted in the 12-24 hour range, and NPH insulins are still in wide use today. Currently this type of insulin is labelled N.
Lente insulins are no longer in use; this next section will tell you why.
In the 1950s, Hallas-Møeller and colleagues released a set of insulin preparations known as insulin lente (semi-lente, lente, and ultra-lente). They added zinc to neutral insulin (porcine and bovine) to create longer durations. Ultra-lente was the first “long-acting” insulin and was used much like insulin glargine and insulin detemir are used today.
One of the reasons we don’t see the lente insulins anymore is that human insulin has different amino acid sequences and therefore, has a shorter duration of effect than both bovine and porcine insulins. This meant the process for preparing lente human insulins had to be different from the lente animal insulins. Turns out that the process was so different that the lente human insulins just did not work well. (BTW, they already knew about variations between species; ultra-lente was only bovine-sourced since that process did not work well with porcine-sourced insulin. Lente insulins left the market in the 1990s.)
Jump to 1978.
Genetically engineered bacteria/yeast with human insulin genes inserted in them could now produce human insulin. In 1982, this human insulin came on the market as “Humulin” from Eli Lilly and company. The advantage was that it was less immunogenic and had less lipodystropy at the injection site than the animal products. It could be made with an NPH formulation for intermediate action, and the soluble human insulin could be mixed with the suspended NPH in a single injection. This regular human insulin was labelled R.
Read more history here: https://www.liebertpub.com/doi/pdfplus/10.1089/dia.2011.0068
By the 1990s, human insulins were almost solely in use. Porcine and bovine insulins would only be on the market until 2003 (and have not been missed).
A faster onset insulin was the next target.
How Did We Get Rapid-Onset Insulins?
Several insulin analogs according to structural modifications. (Image)
In order to decrease post-prandial glucose spikes, patients had to take their human insulin about 30 minutes before they ate. This could be problematic, especially if someone missed a meal but had already taken insulin.
The first analog of human insulin was insulin aspart, again by Eli Lilly and company. This time it was not the preparation that was changed but the insulin. The gene coding for the insulin B-chain was modified to allow for more rapid absorption like endogenous insulin. Rapid here means an onset within 15 min!
Insulin lispro transposes the proline and lysine amino acids in positions 28 & 29 of the beta-chain.
Similarly, insulin aspart has an aspartic acid in the B-28 position instead of proline.
Insulin glulisine has substitutions at B-3 with lysine and B-29 with glutamic acid.
A longer discussion is discussed by Irl B Hirsch in a 2005 NEJM review: Insulin Analogues.
What about the Long-Acting Insulins?
Once they figured out a rapid-onset insulin, the need for a long-acting insulin was back since ultra-lente was gone. GLARGINE. One word. This insulin was a game-changer.
The short acting and intermediate acting insulins had two major problems: 1) a peak effect and 2) limited duration. Insulin glargine had no peak and a long (24 hour) duration of effect. The popular basal-bolus dosing strategy could work with fewer injections and actually work better since the basal insulin was glargine with no peak as opposed to NPH insulin with a roughly (and variable) 6-hour peak.
Glargine was introduced in the US in 2000. It has substitutions on both the A chain (one) and on the B chain (2 substitutions). It makes the molecule more soluble at an acidic pH by changing the pKa to 6.7. So, in the vial (slightly acidic), glargine is soluble, but at physiologic pH (7.4), it is less soluble and aggregates form. Like the hexamers discussed before, these slowly degrade and enter the circulation. The important difference here is that (unlike NPH) there is no peak, and the release is virtually constant.
There are three products on the market: Lantus U-100, Basaglar U-100, and Toujeo U-300. U-100 refers to 100 units/mL, U-300 means 300 units/mL (aka more concentrated). The U-100 and U-300 products are NOT interchangeable. And while the manufacturers of Lantus and Basaglar do not state the U-100 products are interchangeable, functionally, they are. BUT if the RX was written for the BRAND NAME, a new prescription would be needed.
Sidebar: What’s with all the different insulin concentrations?
Insulins have come in different concentrations over the years, but dosing errors made it necessary to standardize. For a number of years, the only concentration of any insulin was 100 units in one milliliter, aka U-100. Since T2DM is primarily a disease of insulin resistance, high doses of insulin are often required, necessitating large volumes of insulin solutions/suspensions.
The largest insulin syringes on the market will only hold one milliliter (1 mL). So any dose over 100 units required more than one “stick.” By concentrating the insulin to U-200, U-300, or even U-500, smaller volumes can provide higher doses of insulin. But at a price…. Dosing errors.
To combat this, the makers of the U-200 and U-300 products only sell the product in a pen-device. The patient dials the dose in units and never has to deal with the volume. However, U-500 is available in both a pen-device and in vials. We strongly suggest only writing prescriptions for the pen device for obvious safety reasons. HOWEVER, the pen has not been on the market for very long, and many patients do still use vials. Hospitals frequently use the vial and have the pharmacist draw up the dose and send it to the floor (whereas most facilities have the nurse draw up U-100 insulins on the floor).
Again, there is a problem. While U-100 syringes are available with safety shield devices to prevent inadvertent needle sticks by staff, the U-500 syringes on the market do not. Some hospital policies mandate all injections will be with safety devices; this puts the nurses’ safety (avoiding a needle stick) above the patient safety (avoiding dosing errors). The solution is often to use a safety shielded U-100 syringe to draw up and inject U-500 insulins. ARRRGGHHH, 5-fold dosing errors abound! (See this U-500 blog post for more.)
Anyways, back to the long-acting insulins. Next we discuss insulin detemir.
Like human insulin other insulin analogs, detemir is produced by a recombinant DNA technology. This time, however, the mechanism for extended duration is different. At the amino terminus of the B-chain, detemir has a saturated fatty acid (14 carbon) attached, and the amino acid threonine in position B30 has been eliminated. The fatty acid has an affinity for albumin. There is little subcutaneous albumin, but it is abundant in the blood, on the order of 4 gm/dL.
So, if we think of the subcutaneous injection as providing a depot for most insulins, for detemir the depot would actually be the circulating albumin in the blood. This begs the question - what happens in hypoalbuminemia?
The package insert says that 98% of the detemir is albumin-bound, and no mention of hypoalbuminemia is included. From experience managing patients on detemir, if they are getting higher doses (single doses >40 units daily), then hypoalbuminemia can lead to faster absorption, earlier peaks, and resulting hypoglycemia. It also seems that lower doses (single doses <20 units daily) can also lead to hypoglycemia. Generally, this means we suggest twice daily dosing with insulin detemir. To be fair, the package insert does recommend evening dosing, and you could use a single evening dose if being used in T2DM and oral anti-diabetic agents are being used.
On to our last analog of insulin, degludec.
Insulin degludec has the amino acid at B30 eliminated and is conjugated to hexadecanedioic acid (a 16 carbon fatty acid) via gamma-L-glutamyl spacer at the amino acid lysine at position B29. So, what is unique about degludec?
The unique structure allows for “multihexamers” to be formed. While other insulins form hexamers that must be degraded into monomers before “free” or “active” insulin to be present, degludec aggregates the hexamers. Note that aggregates is our word for this multihexamer formation… But essentially, the multihexamer must breakdown into hexamers, and the hexamers then must dissociate into dimers and then monomers before “free” (aka “active”) insulin is released.
This multistep degradation gives degludec a very long duration of activity with no peak effect, up to 42 hours. The formation of multihexamers is also thought to prevent what is often called “dose stacking” when multiple doses are given.
Sidebar: Why do we use subcutaneous insulin injections?
Since insulin is a protein, it will be degraded by stomach acid, so the oral route is out. And how many people would be interested in a rectal route (eww). So with both enteral routes out of the picture, the parenteral routes are next.
Inhaled insulins have had limited success. IV gives rapid distribution, but frankly is not a good idea for patients’ home use even though it can be used in critical care situations.
That left IM and SQ. As we’ve discussed, it turns out that when given subcutaneously, insulins form insoluble hexamers in the SQ space. These will slowly degrade, and the monomers can be picked up by the bloodstream in the SQ capillaries and then distributed thorough out the body. By tweaking the amino acids (primarily on the B-chain that are responsible for the hexamer formation), the hexamers can degrade into monomers faster… thus we get the rapid-acting insulins. We have three now: lispro, aspart and glulisine; Brands: Humalog, Admelog, Novolog, and Apidra. Lispro is available as a generic and two different brands. Aspart is also available with an additive designed to speed up absorption.
TL;DR Summary of the Insulin Road Trip
Original insulin products were bovine and porcine extracts, which hit the market in the 1920s. They left the market completely in 2003.
NPH insulin is insulin combined with protamine to stabilize the hexamer formation and give a longer duration of action. (NPH = N = intermediate acting. It is a twice daily “basal” insulin.
Recombinant DNA technology gave rise to human insulin entering the market in 1982. (Human insulin = R = Regular). It is a pre-meal (aka pre-prandial) insulin.
The first insulin analogs were the rapid-acting class (lispro, aspart, and glulisine). These are meal-time (aka prandial) insulin.
The first long-acting insulin was insulin glargine, which is a solution - other insulins up to this point are suspensions. When injected subcutaneously, it forms large aggregates and has no peak. This is a once daily basal insulin.
Insulin detemir has a different mechanism (C14 fatty acid) than glargine and has a small peak; the manufacturer says “no pronounced peak.” I guess the peak is silent? This is a daily or twice-daily basal insulin.
Insulin degludec makes multihexamers, which lead to a longer duration (up to 42 hours) but does not “stack”. So dosing daily is not problematic. It is a once daily basal insulin, but exact timing is not as important as in NPH, glargine, and detemir.
Most insulins come as U-100. But some do come as U-200, U-300, and even U-500, primarily to reduce injection volume in insulin-resistant diabetes (T2DM). PAY ATTENTION as the differing concentrations can be a HUGE source of medication dosing errors!!
How to Combine Insulins
This really is three topics.
Using different insulins in a single patient
Premixed insulins in vials or pens
Mixing insulins in syringes from vials
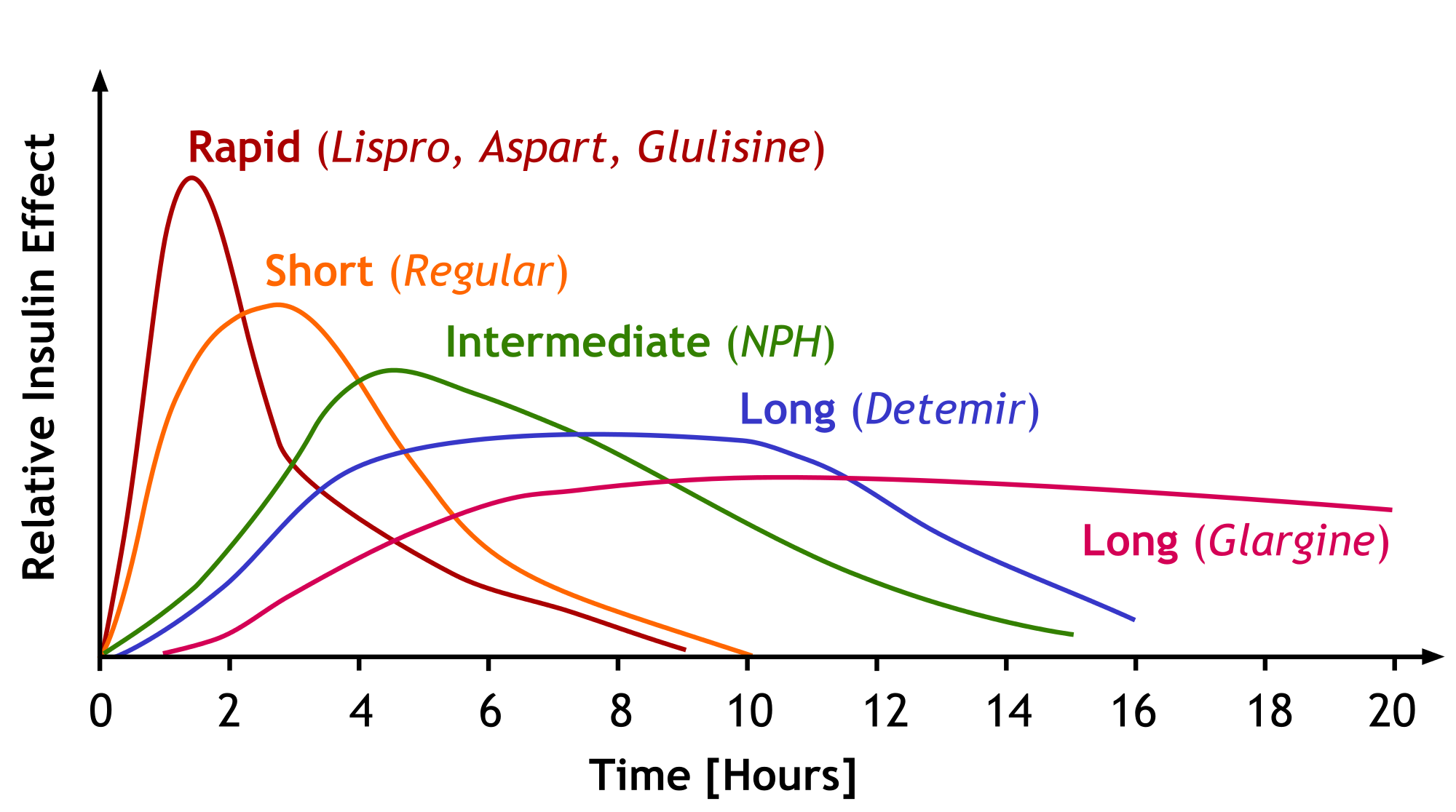
The different insulins clearly have different pharmacokinetic profiles - that is what the medicinal chemistry and pharmaceutics discussed above were all about. If we look at a graph of the different insulins’ effects vs. time, we will understand it better. The image below does not include degludec, but just imagine it as a longer version of the glargine curve. Also realize these are population averages and NOT exactly what your patient is going to do with these insulins.
Learn this. Soak it in. (Image)
This is just page ONE. Seriously, go HERE for the full version and print it. Put it under your pillow and absorb it by osmosis. Thank you, Dr. Barnes!
If we return to how a normal pancreas releases insulin, it should remind us that there is a constant release of insulin that we call a basal release. And with meals there is a release that is related to the carbohydrate intake - a bolus or a prandial or mealtime release. Strategies that try to mimic this are called basal-bolus regimens.
Most basal-bolus regimens try to provide about ½ of the total daily dose of insulin (TDD) as a basal insulin and then the remaining with meals. So, we usually split the bolus doses based on the number of meals per day. Let’s take an example patient who is getting about 100 units daily. We would expect that patient to get 50 units of a long-acting insulin and about 50 units of bolus insulin with meals. This would come out to about 16 or 17 units with each of the three meals a day. Notice we said “about” and not “exactly.”
If we are using glargine, then the basal could be a single dose anytime of the day. If it were NPH or detemir, we would suggest splitting it ½ or 2/3 in the morning and the remainder in the evening.
For most patients, it would not matter which rapid-acting insulin we choose. Any could be used, and it would be dosed as the patient began his/her meal. If Regular insulin was used, it would be best if the dose was given about 30 minutes before the meal based on the PK curve and onset.
Of course, this could mean as many as five injections a day! While some patients do not mind this, others are unhappy with this number and want to reduce it. There are two ways to reduce it (we already mentioned them above) - mix two insulins in the same syringe or use a premixed insulin. Or really, there are three ways with the third being progression to an insulin pump - more here.
Some rules about mixing insulins in a single syringe:
Long-acting and concentrated insulins cannot be mixed with other insulins. NPH formulations can be mixed. Please see the accompanying table for details.
Inject air into each vial equivalent to the volume of insulin you are going to remove before withdrawing any insulin.
Regular insulin or rapid-acting insulins should be drawn into the syringe before intermediate (NPH) insulin. REMEMBER: Clear before cloudy insulin! This prevents the protamine from entering the other vial and potentially affecting the PK parameters of future rapid/regular doses.
Currently available NPH and short-acting insulin formulations when mixed may be used immediately or stored for future use, but most clinicians recommend for immediate use only. (BTW, the same ADA document that says it is ok to store them, also says to use within 15 minutes.)
If lente insulins ever return to the US market, they should not be mixed with NPH or rapid acting insulins.
Considering the complexity of mixing insulins, it is probably a better idea for patients to use a premixed insulin if that is the route you want to go with the patient. The currently available products are:
50% lispro N/50% lispro also called 50% lispro protamine/50% lispro (Humalog Mix 50/50)
75% lispro N/25% lispro also called 75% lispro protamine/25% lispro (Humalog Mix 75/25)
70% aspart N/30% aspart also called 70% aspart protamine/30% aspart (Novolog Mix 70/30)
70% NPH/30% regular (Novolin 70/30 and Humulin 70/30)
When to use these premixed insulin products?
When a patient wants to minimize the number of injections daily and understands that glucose control may not be as tight as with a typical basal bolus regimen.
When a patient cannot take a “midday” dose because insulin storage is not possible.
Let’s look at an example. Let’s say you, like Dr. Barnes, practice in Texas. Summer time gets hot! If you have a patient who works outside and has no access to refrigeration during the day to store his insulin safely, you might use a 70/30 or 75/25 insulin mixture.
Let us assume the patient gets 50 units of insulin daily. When using 70/30, we generally give 2/3 of the total daily dose with the morning meal and 1/3 with the evening meal. Two-thirds of 50 would be 33 units of the 70/30 in the morning with the remaining 17 units (50 minus 33) in the evening.
That 33 units of 70/30 in the morning would be equivalent to giving 23 units of NPH (70% of 33 units) and 10 units (30% of 33 units) of regular insulin in a single injection. The Regular insulin will cover the carbohydrates in the morning meal, and the NPH will act as half of the daily basal dose and also help to somewhat cover the noontime meal.
Then the other third of his total daily dose (17 units of 70/30) given in the evening will provide about 11.5 units (70% of 17 units) of NPH for the overnight basal coverage and almost 5 units (30% of 17 units) of regular to cover the evening meal.
This means the patient gets two injections daily, does not have to carry insulin to the workplace, does not have to worry about storage outside the home, AND gets both a basal insulin (NPH) and at least two meals with bolus insulin coverage. Granted, it also means he misses a midday meal bolus dose, and we have to work within the confines of the fixed 70/30 ratio when determining how much bolus he’ll get with his morning and evening meals.
While this is not ideal, neither is having uncontrolled diabetes due to incorrectly stored insulin or non adherence due to multiple injections. So, in reality, this is a lot better than a patient not using other regimens properly or at all.
Depending on cost (and insurance coverage), the 75/25 mixes can be used the same way. Honestly, there’s rarely a need to use 50/50, but it has been used at the evening meal with 70/30 for the morning meal. It means the patient has to buy two different mixtures, but if the patient skips lunch and has a large evening meal, this can work.
How to Start Insulin Therapy
T1DM. Doses usually range around 0.4-0.5 units/kg of total body weight. Generally, 40-50% of the total daily dose of insulin is given as a long-acting insulin. The package inserts for these products (Lantus®, Basaglar®, Levemir®, and Tresiba®) suggest 1/3 of the expected total daily dose of insulin be given as a starting basal dose. No data supports if this should be one or two doses, we suggest starting with one dose and adjusting both the schedule and dose based on patient response.
Since these are long-acting insulins and stacking is possible, for outpatients, you would not change the dose any faster than weekly (according to experience, not data - the package insert for Tresiba® says to adjust every 3-4 days). Carefully monitored inpatients may be able to be titrated more quickly.
The balance of the insulin daily dose should be divided based on meals. (Remember, patients with T1DM are insulin-dependent. In order to most accurately mimic natural insulin physiology, a basal/bolus regimen will be necessary right out of the gate.) Be sure to UNDERDOSE rather than overdose the insulin to avoid hypoglycemia.
Guidelines suggest that the long-acting insulin analogs are preferred to NPH. They clearly state that there is no evidence of lower HbA1c with the analogs but that there is a “consistent” reduction in hypoglycemic events. Recent real-world data suggests that emergency room visits for hypoglycemia are no different between the types of insulin initiation.
Billy Madison knows the struggle.
Prandial insulin doses are estimated best by looking at the carbohydrate (CHO) content of the meal, aka “carb counting.” Typically, we count 15 grams of carbohydrates as “one carb.” And then give one unit of insulin per “carb.” But for patients extremely sensitive to insulin, this may be 1 unit of insulin per 20 grams of CHO, whereas insulin-resistant patients may need 1 unit of insulin per 5 grams of CHO.
An insulin sensitivity factor (ISF) can be calculated; the formula usually used is:
1,800/TDD = number of mg/dL of glucose that will be reduced by 1 unit of insulin.
If you have a patient struggling with needle burden, you might consider an insulin pump. These devices use a catheter to deliver rapid-acting insulin, which means the patient doesn’t have to inject multiple times a day. The pump can be programmed to deliver both continuous and bolus insulin throughout the day and around meals, respectively. Although a needle is still involved with the pumps, the newer pumps use a very tiny needle! Check out this post for a full explanation about insulin pumps.
T2DM. Both major guidelines suggest insulin for T2DM when a patient has an HbA1c> 9.0% whether they are insulin naïve or not, or if not making progress on oral medications. The ADA’s 2019 guideline for intensifying injectable therapies is here. The AACE algorithm is copyrighted, so we will link to it rather than show you directly. While they vary slightly, the gist of the both algorithms is to start a basal insulin based on HbA1c. Per AACE, if HbA1c is greater than 8%, use 0.2-0.3 units/kg. If less than 8%, the starting dose should be 0.1-0.2 units/kg. Then titrate every 2-3 days (again we caution about being too aggressive if unable to monitor the patient).
Alternatively, the package inserts for all three long-acting insulins suggest a starting dose of 10 units daily or nightly. This may be a good strategy for truly naïve patients in whom you have no idea of the insulin sensitivity.
BTW, the only “non-insulin” agent FDA-approved for T1DM is pramlintide. This injection is co-administered with prandial insulin. It reduces postprandial glucose increases via the following mechanisms: prolongation of gastric emptying time, reduction of postprandial glucagon secretion, and reduction of caloric intake through appetite suppression.
Oral Medications as Adjuncts to Insulin
The AACE algorithm mentioned above suggests intensifying insulin if the T2DM patient does not reach goals on basal insulin. Specifically, they suggest GLP-1 receptor agonists, SGLT-2 inhibitors, or DPP4 inhibitors. See this post, and also stay tuned for Part 2 of this series to learn more about oral anti-diabetic agents.
The Money Talk
Ok, now we want to ask some questions.
You now know the PK advantages of the newer analog insulins over human Regular insulin and NPH.
Knowing these PK advantages, who would use the “old” insulins? Wouldn’t it be malpractice to use these old ones in the face of the advantages of the new ones?
Have you looked at the cost of the analogs? Whew!!! Some are 10 times the price of Regular. Tell us which is better… A Rx for an analog that is not filled or Regular/NPH that is used?
But don’t just take our word for this. There are actually good studies that point out that NPH is non-inferior to the other intermediate and long-acting insulin analogs when it comes to some important health outcomes. The data for analog superiority is weak. It is somewhat better in T1DM compared to T2DM. Take a look here, and listen to this podcast with Dr. Lipska to understand this more.
If we understand the literature correctly, the British NHS prefers NPH to glargine/detemir/degludec. If we were an insurance payer like NHS trying to maximize our patient outcomes, there’d probably be a push for NPH. For the price of one patient on an analog, we could treat 10 patients with NPH and have the same outcomes.
Medical inertia is hard to overcome. “Noninferior” is not the same as “superior”, and if the prescribers and patients perceive an advantage, they may still choose the more expensive analog over human insulin.
We Need to Chat Hypoglycemia
So about this hypoglycemia we mentioned…
Now that we’ve talked about insulin, it’s important to know that the most important side effect of insulin is hypoglycemia. *audible gasp* What does this mean?
Hypoglycemia occurs when there is too little glucose in your body, and it is usually defined as a blood glucose <70 mg/dL. It can happen if you give too much insulin, if you give your normal amount of insulin but didn’t eat at all, or even out of the blue.
What does it look like? Hypoglycemia can present itself as lightheadedness, shaking, fainting, sweating, irritability, etc. (Important note: these symptoms, except for sweating, may be masked if a patient is on a beta blocker.)
Find a source of sugar that will help bring their blood glucose level back to normal. DO NOT GIVE THEM CHOCOLATE. Chocolate and other high-fat sugar sources are not helpful in emergency situations because the fats can actually slow down glucose absorption.
An easy way to remember what is a-okay to give someone experiencing a hypoglycemic episode is the Rule of 15: consume 15 grams of carbohydrate, wait about 15 minutes, then re-check the blood glucose level (if a glucose meter is easily accessible).
The following items contain 15 grams of carbohydrate:
3–4 glucose tablets
1 dose of glucose gel (in most cases, 1 small tube is one dose)
1/2 cup of orange juice or regular soda (not sugar-free)
1 tablespoon of honey or syrup
1 tablespoon of sugar or 5 small sugar cubes
6–8 LifeSavers
8 ounces of skim (nonfat) milk
After they get back on their feet, it’s advised to recheck their blood glucose again in about an hour since blood glucose levels can begin to drop again within that time frame.
Hypoglycemia may even cause patients to lose consciousness or to (hopefully not) have seizures, especially when blood glucose is lower than 50 mg/dL. No need to grab the AED just yet (anti-epileptic drug OR automated external defibrillator…so many acronyms)!
Instead, administer a glucagon injection if you have it and call 911. Glucagon will cause their liver to release sugar, and the patient should recover within 15 minutes or so --- re-check their blood sugar to be sure it rises above 70 mg/dL!
Trending Topic: Did you know there’s a new intranasal glucagon that’s supposed to hit the market soon-ish (fingers crossed by the end of 2019)? Unlike the glucagon injection, this ready-to-use dry powder spray does not require reconstitution. Before you ask, nasal congestion does not impact its efficacy!
Another pharmacist intervention: check for opportunities to lower doses or discontinue insulin, sulfonylureas, or other medications that cause hypoglycemia. Prescribe and counsel on glucagon injection or nasal spray in case this happens again!
Whew! So that’s insulin. Hopefully you feel you have a better grasp on the options available and how to use them alone or in combinations to meet your individual patients’ needs! Stay tuned for the next post to learn more about considerations and strategies for piecing together regimens with oral and non-oral medications.