Differentiation in Aquatic Metabolism between Littoral Habitats with Floating-Leaved and Submerged Macrophyte Growth Forms in a Shallow Eutrophic Lake
Abstract
:1. Introduction
2. Materials and Methods
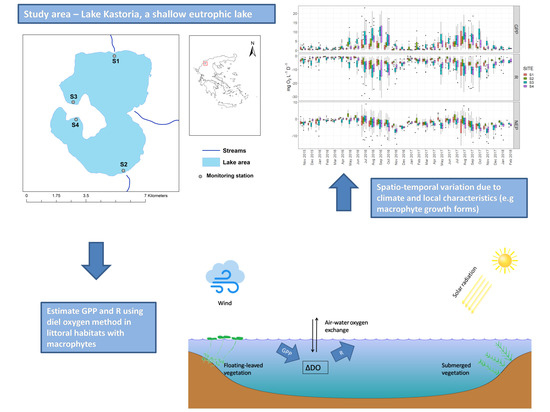
2.1. Site Description
2.2. Collection of Data
2.3. Estimation of Metabolic Balance
2.4. Statistical Analysis
3. Results
3.1. Spatiotemporal Dynamics of Environmental Parameters
3.2. Temporal Dynamics of Metabolic Estimates
3.3. Spatial Differences in Metabolic Estimates
3.4. Exploring Relationships between Metabolic Estimates and Environmental Variables
4. Discussion
4.1. Daily vs. Monthly Variation in Metabolic Estimates
4.2. Spatial and Temporal Heterogeneity in Environmental Variables
4.3. Drivers of Temporal Variation in Metabolism
4.4. Drivers of Spatial Heterogeneity in Metabolism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Odum, H.T. Primary Production in Flowing Waters. Limnol. Oceanogr. 1956, 1, 102–117. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Cole, J.J.; Prairie, Y.T. The study of carbon in inland waters-from isolated ecosystems to players in the global carbon cycle. Limnol. Oceanogr. Lett. 2018, 3, 41–48. [Google Scholar] [CrossRef]
- Staehr, P.A.; Testa, J.M.; Kemp, W.M.; Cole, J.J.; Sand-Jensen, K.; Smith, S.V. The metabolism of aquatic ecosystems: History, applications, and future challenges. Aquat. Sci. 2012, 74, 15–29. [Google Scholar] [CrossRef]
- Tank, S.E.; Fellman, J.B.; Hood, E.; Kritzberg, E.S. Beyond respiration: Controls on lateral carbon fluxes across the terrestrial-aquatic interface. Limnol. Oceanogr. Lett. 2018, 3, 76–88. [Google Scholar] [CrossRef]
- Hanson, P.C.; Bade, D.L.; Carpenter, S.R.; Kratz, T.K. Lake metabolism: Relationships with dissolved organic carbon and phosphorus. Limnol. Oceanogr. 2003, 48, 1112–1119. [Google Scholar] [CrossRef]
- Hanson, P.C.; Carpenter, S.R.; Armstrong, D.E.; Stanley, E.H.; Kratz, T.K. Lake dissolved inorganic carbon and dissolved oxygen: Changing drivers from days to decades. Ecol. Monogr. 2006, 76, 343–363. [Google Scholar] [CrossRef]
- Brighenti, L.S.; Staehr, P.A.; Luciana, L.P.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Importance of nutrients, organic matter and light availability on epilimnetic metabolic rates in a mesotrophic tropical lake. Freshw. Biol. 2018, 63, 1143–1160. [Google Scholar] [CrossRef]
- Staehr, P.A.; Bade, D.; van de Bogert, M.C.; Koch, G.R.; Williamson, C.; Hanson, P.; Cole, J.J.; Kratz, T. Lake metabolism and the diel oxygen technique: State of the science. Limnol. Oceanogr. Methods 2010, 8, 628–644. [Google Scholar] [CrossRef]
- Staehr, P.A.; Baastrup-Spohr, L.; Sand-Jensen, K.; Stedmon, C. Lake metabolism scales with lake morphometry and catchment conditions. Aquat. Sci. 2012, 74, 155–169. [Google Scholar] [CrossRef]
- Brighenti, L.S.; Staehr, P.A.; Gagliardi, L.M.; Brandão, L.P.M.; Elias, E.C.; de Mello, N.A.S.T.; Barbosa, F.A.R.; Bezerra-Neto, J.F. Seasonal Changes in Metabolic Rates of Two Tropical Lakes in the Atlantic Forest of Brazil. Ecosystems 2015, 18, 589–604. [Google Scholar] [CrossRef]
- Hotchkiss, E.R.; Sadro, S.; Hanson, P.C. Toward a more integrative perspective on carbon metabolism across lentic and lotic inland waters. Limnol. Oceanogr. Lett. 2018, 3, 57–63. [Google Scholar] [CrossRef]
- Seekell, D.A.; Lapierre, J.-F.; Cheruvelil, K.S. A geography of lake carbon cycling. Limnol. Oceanogr. Lett. 2018, 3, 49–56. [Google Scholar] [CrossRef]
- Staehr, P.A.; Sand-Jensen, K. Temporal dynamics and regulation of lake metabolism. Limnol. Oceanogr. 2007, 52, 108–120. [Google Scholar] [CrossRef]
- Laas, A.; Nõges, P.; Kõiv, T.; Nõges, T. High-frequency metabolism study in a large and shallow temperate lake reveals seasonal switching between net autotrophy and net heterotrophy. Hydrobiologia 2012, 694, 57–74. [Google Scholar] [CrossRef]
- Solomon, C.T.; Bruesewitz, D.A.; Richardson, D.C.; Rose, K.C.; Van de Bogert, M.C.; Hanson, P.C.; Kratz, T.K.; Larget, B.; Adrian, R.; Leroux Babin, B.; et al. Ecosystem respiration: Drivers of daily variability and background respiration in lakes around the globe. Limnol. Oceanogr. 2013, 58, 849–866. [Google Scholar] [CrossRef]
- Van de Bogert, M.C.; Bade, D.L.; Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Hanson, P.C.; Langman, O.C. Spatial heterogeneity strongly affects estimates of ecosystem metabolism in two north temperate lakes. Limnol. Oceanogr. 2012, 57, 1689–1700. [Google Scholar] [CrossRef]
- Lauster, G.H.; Hanson, P.C.; Kratz, T.K. Gross primary production and respiration differences among littoral and pelagic habitats in northern Wisconsin lakes. Can. J. Fish. Aquat. Sci. 2006, 63, 1130–1141. [Google Scholar] [CrossRef]
- Van de Bogert, M.C.; Carpenter, S.R.; Cole, J.J.; Pace, M.L. Assessing pelagic and benthic metabolism using free water measurements. Limnol. Oceanogr. Methods 2007, 5, 145–155. [Google Scholar] [CrossRef]
- Sadro, S.; Melack, J.M.; MacIntyre, S. Spatial and Temporal Variability in the Ecosystem Metabolism of a High-elevation Lake: Integrating Benthic and Pelagic Habitats. Ecosystems 2011, 14, 1123–1140. [Google Scholar] [CrossRef]
- Vesterinen, J.; Devlin, S.P.; Syväranta, J.; Jones, R.I. Influence of littoral periphyton on whole-lake metabolism relates to littoral vegetation in humic lakes. Ecology 2017, 98, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Idrizaj, A.; Laas, A.; Anijalg, U.; Nõges, P. Horizontal differences in ecosystem metabolism of a large shallow lake. J. Hydrol. 2016, 535, 93–100. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton food web structure in a eutrophic polymictic lake with a history in toxic cyanobacterial blooms. Limnol. Oceanogr. 2006, 51, 715–727. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papastergiadou, E. Influence of hydrophyte abundance on the spatial distribution of zooplankton in selected lakes in Greece. Hydrobiologia 2010, 656, 55–65. [Google Scholar] [CrossRef]
- Stefanidis, K. Ecological Assessment of Lakes of NW Greece with Emphasis on the Associations between Aquatic Macrophytes, Zooplankton and Water Quality. Ph.D. Thesis, Department of Biology, University of Patras, Patras, Greece, 2012. [Google Scholar] [CrossRef]
- Stefanidis, K.; Papastergiadou, E. Relationships between lake morphometry, water quality and aquatic macrophytes in Greek lakes. Fresenius Environ. Bull. 2012, 21, 3018–3026. [Google Scholar]
- Moustaka-Gouni, M.; Vardaka, E.; Tryfon, E. Phytoplankton species succession in a shallow Mediterranean lake (L. Kastoria, Greece): Steady-state dominance of Limnothrixredekei, Microcystis aeruginosa and Cylindrospermopsisraciborskii. Hydrobiologia 2007, 575, 129–140. [Google Scholar] [CrossRef]
- Latinopoulos, D.; Ntislidou, C.; Kagalou, I. A multi-approach Lake Habitat Survey method for impact assessment in two heavily modified lakes: A case of two Northern Greek lakes. Environ. Monit. Assess. 2018, 190, 658. [Google Scholar] [CrossRef] [PubMed]
- Winslow, L.A.; Zwart, J.A.; Batt, R.D.; Dugan, H.A.; Iestyn Woolway, R.; Corman, J.R.; Hanson, P.C.; Read, J.S. LakeMetabolizer: An R package for estimating lake metabolism from free-water oxygen using diverse statistical models. InlandWaters 2016, 6, 622–636. [Google Scholar] [CrossRef]
- Woolway, R.I.; Jones, I.D.; Hamilton, D.P.; Maberly, S.C.; Muraoka, K.; Read, J.S.; Smyth, R.L.; Winslow, L.A. Automated calculation of surface energy fluxes with high-frequency lake buoy data. Environ. Model. Softw. 2015, 70, 191–198. [Google Scholar] [CrossRef]
- Vachon, D.; Prairie, Y.T. The ecosystem size and shape dependence of gas transfer velocity versus wind speed relationships in lakes. Can. J. Fish. Aquat. Sci. 2013, 70, 1757–1764. [Google Scholar] [CrossRef]
- Dugan, H.A.; Iestyn Woolway, R.; Santoso, A.B.; Corman, J.R.; Jaimes, A.; Nodine, E.R.; Patil, V.P.; Zwart, J.A.; Brentrup, J.A.; Hetherington, A.L.; et al. Consequences of gas flux model choice on the interpretation of metabolic balance across 15 lakes. InlandWaters 2016, 6, 581–592. [Google Scholar] [CrossRef]
- Batt, R.D.; Carpenter, S.R. Free-water lake metabolism: Addressing noisy time series with a Kalman filter. Limnol. Oceanogr. Methods 2012, 10, 20–30. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bartón, K. MuMIn: Multi-Model Inference. R Package Version 1.15.6. 2016. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 18 November 2018).
- Rose, K.C.; Winslow, L.A.; Read, J.S.; Read, E.K.; Solomon, C.T.; Adrian, R.; Hanson, P.C. Improving the precision of lake ecosystem metabolism estimates by identifying predictors of model uncertainty. Limnol. Oceanogr. Methods 2014, 12, 303–312. [Google Scholar] [CrossRef]
- Cremona, F.; Laas, A.; Nõges, P.; Nõges, T. An estimation of diel metabolic rates of eight limnological archetypes from Estonia using high-frequency measurements. InlandWaters 2016, 6, 352–363. [Google Scholar] [CrossRef]
- Hanson, P.C.; Carpenter, S.R.; Kimura, N.; Wu, C.; Cornelius, S.P.; Kratz, T.K. Evaluation of metabolism models for free-water dissolved oxygen methods in lakes. Limnol. Oceanogr. Methods 2008, 6, 454–465. [Google Scholar] [CrossRef]
- Andersen, M.R.; Sand-Jensen, K.; Woolway, R.I.; Jones, I.D. Profound daily vertical stratification and mixing in a small, shallow, wind-exposed lake with submerged macrophytes. Aquat. Sci. 2017, 79, 395–406. [Google Scholar] [CrossRef]
- Caraco, N.F.; Cole, J.J. Contrasting impacts of a native and alien macrophyte on dissolved oxygen in a large river. Ecol. Appl. 2002, 12, 1496–1509. [Google Scholar] [CrossRef]
- Caraco, N.; Cole, J.; Findlay, S.; Wigand, C. Vascular Plants as Engineers of Oxygen in Aquatic Systems. Bioscience 2006, 56, 219. [Google Scholar] [CrossRef]
- Goodwin, K.; Caraco, N.; Cole, J. Temporal dynamics of dissolved oxygen in a floating-leaved macrophyte bed. Freshw. Biol. 2008, 53, 1632–1641. [Google Scholar] [CrossRef]
- Yamaki, A.; Yamamuro, M. Floating-leaved and emergent vegetation as habitat for fishes in a eutrophic temperate lake without submerged vegetation. Limnology 2013, 14, 257–268. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; Allende, L.; O’Farrell, I. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J. Plankton Res. 2007, 29, 47–56. [Google Scholar] [CrossRef]
- Staehr, P.A.; Christensen, J.P.A.; Batt, R.D.; Read, J.S. Ecosystem metabolism in a stratified lake. Limnol. Oceanogr. 2012, 57, 1317–1330. [Google Scholar] [CrossRef]
- Alfonso, M.; Brendel, A.; Vitale, A.; Seitz, C.; Piccolo, M.; Perillo, G. Drivers of Ecosystem Metabolism in Two Managed Shallow Lakes with Different Salinity and Trophic Conditions: The Sauce Grande and La Salada Lakes (Argentina). Water 2018, 10, 1136. [Google Scholar] [CrossRef]
- Pinardi, M.; Bartoli, M.; Longhi, D.; Viaroli, P. Net autotrophy in a fluvial lake: The relative role of phytoplankton and floating-leaved macrophytes. Aquat. Sci. 2011, 73, 389–403. [Google Scholar] [CrossRef]
- Cole, J.J.; Pace, M.L.; Carpenter, S.R.; Kitchell, J.F. Persistence of net heterotrophy in lakes during nutrient addition and food web manipulations. Limnol. Oceanogr. 2000, 45, 1718–1730. [Google Scholar] [CrossRef]
- Karlsson, J.; Byström, P.; Ask, J.; Ask, P.; Persson, L.; Jansson, M. Light limitation of nutrient-poor lake ecosystems. Nature 2009, 460, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Prairie, Y.T. Prevalence of heterotrophy and atmospheric CO2 emissions from aquatic ecosystems. Ecosystems 2005, 8, 862–870. [Google Scholar] [CrossRef]
- Hoellein, T.J.; Bruesewitz, D.A.; Richardson, D.C. Revisiting Odum (1956): A synthesis of aquatic ecosystem metabolism. Limnol. Oceanogr. 2013, 58, 2089–2100. [Google Scholar] [CrossRef]
- Horppila, J.; Nurminen, L. Effects of different macrophyte growth forms on sediment and P resuspension in a shallow lake. Hydrobiologia 2005, 545, 167–175. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.M.; Nagengast, B. The influence of the spatial structure of hydromacrophytes and differentiating habitat on the structure of rotifer and cladoceran communities. Hydrobiologia 2006, 559, 203–212. [Google Scholar] [CrossRef]
- Holmroos, H.; Horppila, J.; Niemistö, J.; Nurminen, L.; Hietanen, S. Dynamics of dissolved nutrients among different macrophyte stands in a shallow lake. Limnology 2014, 16, 31–39. [Google Scholar] [CrossRef]
- Alfonso, M.B.; Vitale, A.J.; Menéndez, M.C.; Perillo, V.L.; Piccolo, M.C.; Perillo, G.M.E. Estimation of ecosystem metabolism from diel oxygen technique in a saline shallow lake: La Salada (Argentina). Hydrobiologia 2015, 752, 223–237. [Google Scholar] [CrossRef]









| Lake Information | Value | Source |
|---|---|---|
| Latitude | 40°31′ N | [23] |
| Longitude | 21°18′ E | |
| Altitude (m) | 629 | |
| Retention time (years) | >2 | |
| Mean depth (m) | 4.4 | [24] |
| Max. depth (m) | 9 | |
| Surface (km2) | 28 | |
| Average phosphorus concentration (mg L−1) | 0.2 | [26] |
| Average dissolved inorganic nitrogen concentration (mg L−1) | 0.27 | |
| Average chlorophyll-a concentration (μg L−1) | 15.90 | |
| Aquatic vegetation (most common species) | Trapa natans, Myriophyllum spicatum, Ceratophyllum demersum | [25,26] |
| Site | Aquatic Vegetation | Adjacent Land Use | Lakeshore Morphological Alterations |
|---|---|---|---|
| S1 | Submerged vegetation (Myriophyllum spicatum, Ceratophyllum demersum) | Agricultures | Low–Moderate |
| S2 | Agricultures | Moderate | |
| S3 | Floating-leaved vegetation (Trapa natans) | Urbanized land uses | High High |
| S4 | Floating-leaved vegetation (Trapa natans) | Urbanized land uses |
| Parameter | Abbreviation | Frequency of Measurement |
|---|---|---|
| Concentration of dissolved oxygen (mg L−1) | DO | 1 h |
| Water temperature (°C) | Wtr | 1 h |
| Air temperature (°C) | airT | 10 min |
| Wind speed at 10 m height (m s−1) | wnd | 10 min |
| Photosynthetically active radiation (W m−2) | irr | 10 min |
| Relative humidity (%) | rh | 10 min |
| pH | pH | 1 h |
| Electrical conductivity (μS cm−1) | cond | 1 h |
| Chlorophyll-a (μg L−1) | chl-a | 1 h |
| GPP | R | NEP | Wnd | Rh | PAR | AirT. | Chl-a | EC | DO | pH | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| R | −0.77 ** | 1 | |||||||||
| NEP | 0.04 | 0.61 ** | 1 | ||||||||
| Wnd | −0.13 ** | 0.04 * | −0.1 ** | 1 | |||||||
| Rh | −0.27 ** | 0.09 ** | −0.19 ** | −0.17 ** | 1 | ||||||
| PAR | 0.38 ** | −0.11 ** | 0.29 ** | 0.07 | −0.77 ** | 1 | |||||
| AirT. | 0.48 ** | −0.25 ** | 0.2 ** | 0.05 | −0.47 ** | 0.67 ** | 1 | ||||
| Chl-a | −0.08 ** | 0.14 ** | 0.12 ** | −0.05 | 0.08 * | −0.09 | −0.07 | 1 | |||
| EC | −0.17 ** | 0.13 ** | −0.01 | 0.2 ** | −0.15 ** | 0.18 ** | −0.05 ** | −0.14 ** | 1 | ||
| DO | −0.22 ** | 0.51 ** | 0.52 ** | 0.08 ** | 0.07 | −0.1 ** | −0.25 ** | 0.16 ** | −0.01 | 1 | |
| pH | 0.4 ** | −0.1 ** | 0.33 ** | 0.03 | −0.23 ** | 0.39 ** | 0.52 ** | 0.1 ** | −0.25 ** | 0.19 ** | 1 |
| Wtr. | 0.53 ** | −0.31 ** | 0.17 ** | −0.01 ** | −0.45 ** | 0.7 ** | 0.91 ** | −0.07 | −0.06 ** | −0.35 ** | 0.54 ** |
| Dependent Variable | Parameter | Pseudo—R2 | Coefficient | p |
|---|---|---|---|---|
| Daily time scale | ||||
| GPPd | (intercept) | 0.544 | −0.304 | p < 0.001 |
| Wtr. | 0.407 | p < 0.001 | ||
| PAR | 0.211 | p < 0.001 | ||
| Wnd | 0.019 | p < 0.001 | ||
| EC | 0.021 | p < 0.001 | ||
| Chl-a | −0.035 | p = 0.086 | ||
| S2 | −0.072 | p = 0.148 | ||
| S3 | 0.839 | p < 0.001 | ||
| S4 | 0.548 | p < 0.001 | ||
| Rd | (intercept) | 0.334 | 0.252 | p < 0.001 |
| PAR | 0.111 | p < 0.001 | ||
| Wtr. | −0.292 | p < 0.001 | ||
| EC | 0.240 | p < 0.001 | ||
| S2 | 0.303 | p < 0.001 | ||
| S3 | −0.846 | p < 0.001 | ||
| S4 | −0.623 | p < 0.001 | ||
| NEPd | (intercept) | 0.170 | 0.003 | p = 0.946 |
| PAR | 0.342 | p < 0.001 | ||
| Wnd | −0.085 | p < 0.001 | ||
| Chl-a | 0.095 | p < 0.001 | ||
| EC | 0.055 | p = 0.039 | ||
| S2 | 0.291 | p < 0.001 | ||
| S3 | −0.201 | p = 0.006 | ||
| S4 | −0.189 | p = 0.010 | ||
| Monthly time scale | ||||
| GPPm | (intercept) | 0.778 | −0.391 | p = 0.001 |
| Wnd | −0.180 | p < 0.001 | ||
| Wtr. | 0.698 | p < 0.001 | ||
| Chl-a | 0.101 | p = 0.112 | ||
| S2 | −0.003 | p = 0.984 | ||
| S3 | 1.067 | p < 0.001 | ||
| S4 | 0.444 | p = 0.005 | ||
| Rm | (intercept) | 0.606 | 0.211 | p = 0.052 |
| Wtr. | −0.988 | p < 0.001 | ||
| PAR | 0.679 | p < 0.001 | ||
| EC | 0.147 | p = 0.052 | ||
| S2 | 0.384 | p = 0.050 | ||
| S3 | −0.943 | p < 0.001 | ||
| S4 | −0.452 | p = 0.021 | ||
| NEPm | (intercept) | 0.401 | −0.007 | p = 0.965 |
| PAR | 1.134 | p < 0.001 | ||
| Wtr. | −0.774 | p < 0.001 | ||
| Wnd | −0.206 | p = 0.069 | ||
| S2 | 0.569 | p = 0.013 | ||
| S3 | −0.314 | p = 0.165 | ||
| S4 | −0.206 | p = 0.361 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanidis, K.; Dimitriou, E. Differentiation in Aquatic Metabolism between Littoral Habitats with Floating-Leaved and Submerged Macrophyte Growth Forms in a Shallow Eutrophic Lake. Water 2019, 11, 287. https://doi.org/10.3390/w11020287
Stefanidis K, Dimitriou E. Differentiation in Aquatic Metabolism between Littoral Habitats with Floating-Leaved and Submerged Macrophyte Growth Forms in a Shallow Eutrophic Lake. Water. 2019; 11(2):287. https://doi.org/10.3390/w11020287
Chicago/Turabian StyleStefanidis, Konstantinos, and Elias Dimitriou. 2019. "Differentiation in Aquatic Metabolism between Littoral Habitats with Floating-Leaved and Submerged Macrophyte Growth Forms in a Shallow Eutrophic Lake" Water 11, no. 2: 287. https://doi.org/10.3390/w11020287