Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review
Abstract
:1. Introduction
2. Material and Methods
2.1. Search Strategy and Selection Criteria
2.1.1. Search Strategy
2.1.2. Identification of Relevant Studies
2.1.3. Types of Study and Design
2.1.4. Population
2.1.5. Quality Assessment/Risk of Bias
2.1.6. Data Extraction and Synthesis
3. Results
3.1. Description of Included Studies
3.2. Quality Assessment
3.3. Relation between NPs and Wound Treatment
3.4. Cytotoxicity Assays
3.5. Antibacterial Response
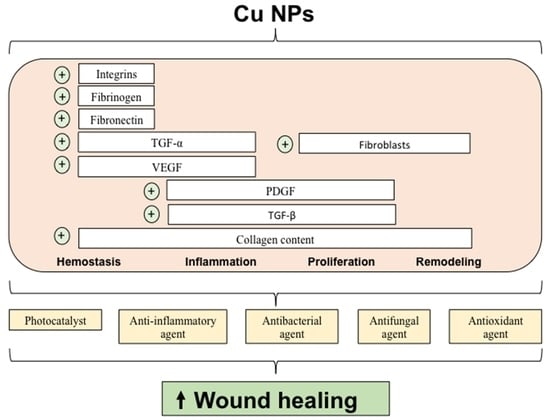
3.6. Wound Healing
4. Discussion
4.1. Summary of Key Findings and Interpretation
4.2. Scope and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Pecoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef]
- Werdin, F.; Tennenhaus, M.; Schaller, H.E.; Rennekampff, H.O. Evidence-based management strategies for treatment of chronic wounds. Eplasty 2009, 9, e19. [Google Scholar]
- Kirsner, R.S. The Wound Healing Society chronic wound ulcer healing guidelines update of the 2006 guidelines—Blending old with new. Wound Repair Regen. 2016, 24, 110–111. [Google Scholar] [CrossRef]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J.; Dardik, R.; Eidelman, A.I.; Lavie, Y.; Grunfeld, Y.; Ikher, S.; Huszar, M.; Zatcoff, R.C.; Marikovsky, M. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen. 2010, 18, 266–275. [Google Scholar] [CrossRef]
- Abdollahi, Z.; Zare, E.N.; Salimi, F.; Goudarzi, I.; Tay, F.R.; Makvandi, P. Bioactive Carboxymethyl Starch-Based Hydrogels Decorated with CuO Nanoparticles: Antioxidant and Antimicrobial Properties and Accelerated Wound Healing In Vivo. Int. J. Mol. Sci. 2021, 22, 2531. [Google Scholar] [CrossRef]
- Ghasemian Lemraski, E.; Jahangirian, H.; Dashti, M.; Khajehali, E.; Sharafinia, S.; Rafiee-Moghaddam, R.; Webster, T.M. Antimicrobial Double-Layer Wound Dressing Based on Chitosan/Polyvinyl Alcohol/Copper: In vitro and in vivo Assessment. Int. J. Nanomed. 2021, 16, 223–235. [Google Scholar] [CrossRef]
- Schencke, C.; Vasconcellos, A.; Sandoval, C.; Torres, P.; Acevedo, F.; del Sol, M. Morphometric evaluation of wound healing in burns treated with Ulmo (Eucryphia cordifolia) honey alone and supplemented with ascorbic acid in guinea pig (Cavia porcellus). Burns Trauma 2016, 4, 25. [Google Scholar] [CrossRef]
- Schencke, C.; Vásquez, B.; Sandoval, C.; del Sol, M. El Rol de la Miel en los Procesos Morfofisiológicos de Reparación de Heridas. Int. J. Morphol. 2016, 34, 385–395. [Google Scholar] [CrossRef]
- Faúndez, G.; Troncoso, M.; Navarrete, P.; Figueroa, G. Antimicrobial activity of copper surfaces against suspensions of Salmonella enterica and Campylobacter jejuni. BMC Microbiol. 2004, 4, 19. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO-cotton nanocomposite: Formation, morphology, and antibacterial activity. Surf. Coat Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Li, Q.; Lu, F.; Zhou, G.; Yu, K.; Lu, B.; Xiao, Y.; Dai, F.; Wu, D.; Lan, G. Silver inlaid with gold nanoparticle/chitosan wound dressing enhances antibacterial activity and porosity, and promotes wound healing. Biomacromolecules 2017, 18, 3766–3775. [Google Scholar] [CrossRef]
- Chen, P.; Bian, L.N.; Hu, X.Y. Synergic fabrication of gold nanoparticles embedded dextran/silk sericin nanomaterials for the treatment and care of wound healing. J. Cluster Sci. 2021, 3, 1. [Google Scholar] [CrossRef]
- Parveen, A.; Kulkarni, N.; Yalagatti, M.; Abbaraju, V.; Deshpande, R. In vivo efficacy of biocompatible silver nanoparticles cream for empirical wound healing. J. Tissue Viability 2018, 27, 257–261. [Google Scholar] [CrossRef]
- Gallo, A.L.; Paladini, F.; Romano, A.; Verri, T.; Quattrini, A.; Sannino, A.; Pollini, M. Efficacy of silver coated surgical sutures on bacterial contamination, cellular response and wound healing. Mater. Sci. Eng. C 2016, 69, 884–893. [Google Scholar] [CrossRef]
- Paladini, F.; De Simone, S.; Sannino, A.; Pollini, M. Antibacterial and antifungal dressings obtained by photochemical deposition of silver nanoparticles. J. Appl. Polym. Sci. 2014, 131, 4032. [Google Scholar] [CrossRef]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface chemical and biological characterization of flax fabrics modified with silver nanoparticles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 1–10. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Maslennikova, V.V.; Konkova, A.A.; Butorova, I.A.; Chistyakov, E.M. Silver-containing hydrogel based on polyvinyl alcohol modified with nanoscale cyclotriphosphazene. Public Health Toxicol. 2021, 1, A23. [Google Scholar] [CrossRef]
- Uauy, R.; Olivares, M.; Gonzalez, M. Essentiality of copper in humans. Am. J. Clin. Nutr. 1998, 67, 952S–959S. [Google Scholar] [CrossRef]
- Tenaud, I.; Sainte-Marie, I.; Jumbou, O.; Litoux, P.; Dreno, B. In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese. Br. J. Dermatol. 1999, 140, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Khanna, S.; Venojarvi, M.; Trikha, P.; Ellison, E.C.; Hunt, T.K.; Roy, S. Copper-induced vascular endothelial growth factor expression and wound healing. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1821–H1827. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, D.; Krans, T.; Espírito Santo, C.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of contact-mediated killing of yeast cells on dry metallic copper surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Ahire, J.J.; Hattingh, M.; Neveling, D.P.; Dicks, L.M. Copper-Containing Anti-Biofilm Nanofiber Scaffolds as a Wound Dressing Material. PLoS ONE 2016, 11, e0152755. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Gabbay, J. Copper as a biocidal tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Alizadeh, S.; Seyedalipour, B.; Shafieyan, S.; Kheime, A.; Mohammadi, P.; Aghdami, N. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem. Biophys. Res. Commun. 2019, 517, 684–690. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.; Yin, W.; Wang, T.; Kang, Y.J. Copper promotes migration of adipose-derived stem cells by enhancing vimentin- Ser39 phosphorylation. Exp. Cell Res. 2020, 388, 111859. [Google Scholar] [CrossRef]
- Das, A.; Sudhahar, V.; Chen, G.F.; Kim, H.W.; Youn, S.W.; Finney, L.; Vogt, S.; Yang, J.; Kweon, J.; Surenkhuu, B.; et al. Endothelial Antioxidant-1: A Key Mediator of Copper-dependent Wound Healing in vivo. Sci. Rep. 2016, 6, 33783. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Kang, Y.J. Copper affects the binding of HIF-1alpha to the critical motifs of its target genes. Metallomics 2019, 11, 429–438. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michopoulou, A.; Rousselle, P. How do epidermal matrix metalloproteinases support re-epithelialization during skin healing? Eur. J. Dermatol. 2015, 25, 33–42. [Google Scholar] [CrossRef]
- Philips, N.; Hwang, H.; Chauhan, S.; Leonardi, D.; Gonzalez, S. Stimulation of cell proliferation and expression of matrixmetalloproteinase-1 and interluekin-8 genes in dermal fibroblasts by copper. Connect. Tissue Res. 2010, 51, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Adamson, I.Y.; Vincent, R.; Bakowska, J. Differential production of metalloproteinases after instilling various urban air particle samples to rat lung. Exp. Lung Res. 2003, 29, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Salvo, J.; Sandoval, C. Role of copper nanoparticles in wound healing for chronic wounds: Literature review. Burns Trauma 2022, 10, tkab047. [Google Scholar] [CrossRef]
- Zhou, M.; Li, J.; Liang, S.; Sood, A.K.; Liang, D.; Li, C. CuS nanodots with ultrahigh efficient renal clearance for positron emission tomography imaging and Image-Guided photothermal therapy. ACS Nano 2015, 9, 7085–7096. [Google Scholar] [CrossRef]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Appendix F Quality Appraisal Checklist—Quantitative Intervention Studies. In Methods for the Development of NICE Public Health Guidance; National Institute for Health and Care Excellence: London, UK, 2012; Available online: https://www.nice.org.uk/process/pmg4/chapter/about-this-document (accessed on 1 April 2022).
- He, W.; Huang, X.; Zheng, Y.; Sun, Y.; Xie, Y.; Wang, Y.; Yue, L. In situ synthesis of bacterial cellulose/copper nanoparticles composite membranes with long-term antibacterial property. J. Biomater. Sci. Polym. Ed. 2018, 29, 2137–2153. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2788. [Google Scholar] [CrossRef]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional Copper-Containing Mesoporous Glass Nanoparticles as Antibacterial and Proangiogenic Agents for Chronic Wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, Y.; Ping, Y.; Zhang, H.; Zhou, B.; Liu, F.; Yu, Y.; Xie, T.; Li, W.; Zhong, D.; Zhang, Y.; et al. Laser-Activatable CuS Nanodots to Treat Multidrug-Resistant Bacteria and Release Copper Ion to Accelerate Healing of Infected Chronic Nonhealing Wounds. ACS Appl. Mater. Interfaces 2019, 11, 3809–3822. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; He, J.; Chen, W.; Yu, Y.; Li, W.; Du, Z.; Xie, T.; Ye, Y.; Hua, S.Y.; Zhong, D.; et al. Light-Activatable Synergistic Therapy of Drug-Resistant Bacteria-Infected Cutaneous Chronic Wounds and Nonhealing Keratitis by Cupriferous Hollow Nanoshells. ACS Nano 2020, 14, 3299–3315. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Thanusha, A.V.; Dinda, A.K.; Koul, V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater. Sci. Eng. C 2018, 89, 378–386. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; van Wachem, P.B.; van Luyn, M.J.; Brouwer, L.A.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. In vitro and in vivo evaluation of gelatin-chondroitin sulphate hydrogels for controlled release of antibacterial proteins. Biomaterials 2000, 21, 1763–1772. [Google Scholar] [CrossRef]
- Wang, P.; Peng, L.; Lin, J.; Li, Y.; Luo, Q.; Jiang, S.; Tian, H.; Zhang, Y.; Liu, X.; Liu, J. Enzyme hybrid virus-like hollow mesoporous CuO adhesive hydrogel spray through glucose-activated cascade reaction to efficiently promote diabetic wound healing. Chem. Eng. J. 2021, 415, 128901. [Google Scholar] [CrossRef]
- Wang, T.L.; Zhou, Z.F.; Liu, J.F.; Hou, X.D.; Zhou, Z.; Dai, Y.L.; Hou, Z.Y.; Chen, F.; Zheng, L.P. Donut-like MOFs of copper/nicotinic acid and composite hydrogels with superior bioactivity for rh-bFGF delivering and skin wound healing. J. Nanobiotechnology 2021, 19, 275. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.; Ameer, G.A. A Cooperative Copper Metal-Organic Framework-Hydrogel System Improves Wound Healing in Diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Xiao, J.; Zhu, Y.; Huddleston, S.; Li, P.; Xiao, B.; Farha, O.K.; Ameer, G.A. Copper Metal-Organic Framework Nanoparticles Stabilized with Folic Acid Improve Wound Healing in Diabetes. ACS Nano 2018, 12, 1023–1032. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, H.; Jiang, Y.; Hua, G.; Zhang, Q.; Zeng, S.; Li, W.; Li, L.; Kang, N.; Ren, L. Nanoliquid Dressing with Enhancing Anti-Infection Performance under the Moderate Photothermal Effect for Wound Treatment. ACS Appl. Mater. Interfaces 2021, 13, 18443–18453. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; van Lith, R.; Baler, K.; Hoshi, R.A.; Ameer, G.A. A Thermoresponsive Biodegradable Polymer with Intrinsic Antioxidant Properties. Biomacromolecules 2014, 15, 3942–3952. [Google Scholar] [CrossRef]
- Djoko, K.Y.; Ong, C.Y.; Walker, M.J.; McEwan, A.G. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef]
- Shariati, A.; Moradabadi, A.; Azimi, T.; Ghaznavi-Rad, E. Wound healing properties and antimicrobial activity of platelet-derived biomaterials. Sci. Rep. 2020, 10, 1032. [Google Scholar] [CrossRef]
- Dev, S.K.; Choudhury, P.K.; Srivastava, R.; Sharma, M. Antimicrobial, anti-inflammatory and wound healing activity of polyherbal formulation. Biomed. Pharmacother. 2019, 111, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: Lessons learned from other inflammatory diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.G.; Felix, F.N.; Woodley, D.T.; Shim, E.K. The role of oxygen in wound healing: A review of the literature. Dermatol. Surg. 2008, 34, 1159–1169. [Google Scholar] [CrossRef]
- Ponugoti, B.; Xu, F.; Zhang, C.; Tian, C.; Pacios, S.; Graves, D.T. FOXO1 promotes wound healing through the up-regulation of TGF-beta1 and prevention of oxidative stress. J. Cell Biol. 2013, 203, 327–343. [Google Scholar] [CrossRef]
- Chandraleka, S.; Ramya, K.; Chandramohan, G.; Dhanasekaran, D.; Priyadharshini, A.; Panneerselvam, A. Antimicrobial mechanism of copper (II) 1,10-phenanthroline and 2,2′-bipyridyl complex on bacterial and fungal pathogens. J. Saudi Chem. Soc. 2014, 18, 953–962. [Google Scholar] [CrossRef]
- O’Gorman, J.; Humphreys, H. Application of copper to prevent and control infection. Where are we now? J. Hosp. Infect. 2012, 81, 217–223. [Google Scholar] [CrossRef]
- Gordon, A.S.; Howell, L.D.; Harwood, V. Responses of diverse heterotrophic bacteria to elevated copper concentrations. Can. J. Microbiol. 1994, 40, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.; Afkhami, F.; Zarei, M.; Ghazvini, K.; Rajabi, O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J. 2014, 40, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B. The challenge of antibiotic resistance. Sci. Am. 1998, 278, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ali, G.W.; Della Sala, F.; Abdel-Fattah, W.I.; Borzacchiello, A. Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohydr. Polym. 2019, 223, 115023. [Google Scholar] [CrossRef]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16, S27. [Google Scholar] [CrossRef]
- Nikolopoulo, G.K.; Paraskevis, D.; Hatzitheodorou, E.; Moschidis, Z.; Sypsa, V.; Zavitsanos, X.; Kalapothaki, V.; Hatzakis, A. Impact of hepatitis B virus infection on the progression of AIDS and mortality in HIV-infected individuals: A cohort study and meta-analysis. Clin. Infect. Dis. 2009, 48, 1763–1771. [Google Scholar] [CrossRef]
- Martins-Green, M.; Saeed, S. Role of Oxidants and Antioxidants in Diabetic Wound Healing. In Wound Healing, Tissue Repair, and Regeneration in Diabetes; Bagchi, D., Das, A., Roy, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–38. [Google Scholar] [CrossRef]
- Wahid, F.; Wang, H.S.; Lu, Y.S.; Zhong, C.; Chu, L.Q. Preparation, characterization and antibacterial applications of carboxymethyl chitosan/CuO nanocomposite hydrogels. Int. J. Biol. Macromol. 2017, 101, 690–695. [Google Scholar] [CrossRef]
- Sattari, S.; Dadkhah Tehrani, A.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. [Google Scholar] [CrossRef]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Uzair, B.; Menaa, F.; Braga, V.A. Fabrication, Physical Characterizations, and In Vitro, In Vivo Evaluation of Ginger Extract-Loaded Gelatin/Poly(Vinyl Alcohol) Hydrogel Films Against Burn Wound Healing in Animal Model. AAPS Pharm. Sci. Tech. 2020, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Mofazzal Jahromi, M.A.; Sahandi Zangabad, P.; Moosavi Basri, S.M.; Sahandi Zangabad, K.; Ghamarypour, A.; Aref, A.R.; Karimi, M.; Hamblin, M.R. Nanomedicine and advanced technologies for burns: Preventing infection and facilitating wound healing. Adv. Drug Deliv. Rev. 2018, 123, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Winey, K.I.; Vaia, R.A. Polymer nanocomposites. MRS Bull. 2007, 32, 314–322. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Ruan, W.H. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Jana, S.; Sen, K.K.; Gandhi, A. Alginate Based Nanocarriers for Drug Delivery Applications. Curr. Pharm. Des. 2016, 22, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles—An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Bibi, S.; Mir, S.; Rehman, W.; Menaa, F.; Gul, A.; Alaryani, F.S.S.; Alqahtani, A.M.; Haq, S.; Abdellatif, M.H. Synthesis and In Vitro/Ex Vivo Characterizations of Ceftriaxone-Loaded Sodium Alginate/poly(vinyl alcohol) Clay Reinforced Nanocomposites: Possible Applications in Wound Healing. Materials 2022, 15, 3885. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of Cephradine-Loaded Gelatin/Polyvinyl Alcohol Electrospun Nanofibers for Effective Diabetic Wound Healing: In-Vitro and In-Vivo Assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]

| References | Country | Population, Setting | Inter Details | Investigated Outcomes | Study Aims | Main Results | |
|---|---|---|---|---|---|---|---|
| [40] | CN | BC cells were used. | BC membranes were divided into 15 mm-diameter rounds. Purified BC membranes were extensively rinsed into CuCl2 solution (20, 60, or 100 mM) to create the BC/Cu composite membranes. | XRD analysis, FTIR spectra, thermal stability, Cu ion release, long-term antibacterial activity, and in vitro cytotoxicity of BC/Cu membranes. | To fabricate BC/Cu composite membrane by in situ chemical reduction method. | Purified BC membranes were first immersed overnight in CuCl2 aqueous solutions, resulting in Cu2+ adhesion to BC nanofibers. Following that, NaBH4 was introduced to the membranes, and the Cu2 anchored in the membranes was instantly reduced to Cu. After 30 min, the reaction was halted, yielding a stable dark-brown BC/Cu membrane. | In BC/Cu membranes: XRD analysis: No differences were found between XRD curves of BC/Cu and cellulose I crystal. FTIR spectra: No differences were found between BC/Cu and BC membranes. Thermal stability, Cu ion release: In BC/Cu100, a considerable weight reduction stage was seen. Antibacterial activity: Significant inhibitions against S. aureus and E. coli after 1, 45, and 90 days were found in all BC/Cu membranes. In vitro cytotoxicity: After being exposed to the membrane extracts from BC/Cu60 and BC/Cu100, cell number was considerably reduced. |
| [41] | CN | HEK293 cells and female BALB/c mice were used. | In vitro: To evaluate ROS scavenging activities and biological compatibility, HEK293 cells were used. In vivo: To evaluate biocompatibility, BALB/c mice were used and 4 μg kg−1 Cu5.4O USNPs were applied. During therapeutic efficacy assessment, BALB/c mice were used in the AKI model, and they were separated as follows: PBS, 8 mg/kg, 40 mg/kg, 160 mg/kg, and they received USNPs at doses of 2 μg/kg, respectively. | In vitro: ROS scavenging activities and biocompatibility of Cu5.4O USNPs. In vivo: Cu5.4O USNPs: biocompatibility and therapeutic efficacy. | To create ultrasmall Cu-based systems, which could serve as a model for future nanosystems employed in the treatment and prevention of disorders connected to ROS. | The Cu5.4O USNPs were created as follows: 10 mM CuCl2 powders in deionized water were dissolved. Next, they were swirled for 10 min at 80 °C. Then, 100 mM L-ascorbic acid solution was added to the CuCl2 solution above, and the pH of the solution was adjusted to 8.0–9.0 using NaOH solution. After the process, the bigger aggregates were removed by centrifugation, and the supernatant was dialyzed against water for two days to remove tiny molecules. Centrifugation was used to concentrate purified Cu5.4O USNPs. | In vitro: ROS scavenging activities of Cu5.4O USNPs: Doses of 150 ng/mL USNPs eliminated most free radicals. Biocompatibility of Cu5.4O USNPs: HEK293 cells showed normal morphology after exposure to 200 ng/mL. USNPs. In vivo: Biocompatibility of Cu5.4O USNPs: No differences between IL-6 and TNF-α levels of USNPs and control group (p > 0.05). Therapeutic efficacy of Cu5.4O USNPs: On days 4, 7, 9, and 15 post-surgery, the wound healing rate was always faster in the USNPs group (p < 0.01). |
| [42] | CA | Human dermal fibroblasts isolated from split-thickness biopsies received from abdominoplasties and breast reduction were used. | Human primary fibroblasts were seeded in DMEM and incubated at 37 °C and 5% CO2. A final concentration of either 0.1 or 1 mg/mL in 1 mL of total suspension of MBG and Cu-MBG was added to the plates. | Cytotoxicity testing, antibacterial effects against planktonic bacteria, antibacterial effects against biofilms, the antibacterial action of Cu-MBG in infected skin, and evaluation of the proangiogenic activity of Cu-MBG. | To evaluate the antibacterial action, both in biofilm models and in the infected tissue-engineered skin model, and to investigate the proangiogenic action using CAM and aortic ring assays. | In double-distilled water, cetyltrimethylammonium bromide and NH4OH solution were dissolved by stirring for 30 min. Next, tetraethyl orthosilicate, Ca(NO3)2·4 H2O, and CuCl2 were dissolved in this solution while stirring for 3 h. Then, the particles were separated by centrifugation at 10,000 rpm for 5 min before being rinsed once with distilled water and again with ethanol. The resulting precipitate was dried for 12 h at 70 °C. Finally, the powders were calcined for five minutes at 600 °C in the air at a heating rate of 1 °C/min. The calcined powders were referred to as Cu-MBG. | Cytotoxicity tests: A reduced cellular metabolic action was found for 1 mg/mL Cu-MBG NPs. No difference for 0.1 mg/mL Cu-MBG was found. Antibacterial effects against planktonic bacteria: Significant effects against P. aeruginosa and S. aureus for 100 μg/mL Cu-MBG were found. Antibacterial effects against biofilms: Reduced biofilms of P. aeruginosa and S. aureus were found after Cu-MBG exposure. Antibacterial action of Cu-MBG in an infected skin: No difference was found in P. aeruginosa-treated samples and controls. Assessment of proangiogenic activity of Cu-MBG: An increased number of junctions, rate of cell outgrowth, area of outgrowth, and total vessel length in Cu-MBG-treated CAM membranes was found. |
| [43] | CN | HFF-1 and HUVEC cells. BALB/c and diabetic mutant (db/db) mice were used. | In vitro: Antibacterial activity: CuS NDs + NIR were used against Escherichia coli and Staphylococcus aureus. Cell migration: HFF-1 cells were used. Cell angiogenesis: HUVECs were incubated in the presence of CuS NDs. In vivo: To develop the infection model, methicillin-resistant Staphylococcus aureus suspension was used. | In vitro: Antibacterial activity, cell migration, and cell angiogenesis. In vivo: Antibacterial activity and wound healing. | To develop CuS NDs stabilized in albumin to improve healing and antibacterial effects. | CuS NDs were created using a simple one-step hydrothermal method. During the production, BSA was utilized to manage particle size and stability. | In vitro: Antibacterial activity: CuS NDs heavily inhibited Escherichia coli and Staphylococcus aureus reproduction. Cell migration: HFF-1 exhibited the greatest migration after CuS NDs+NIR treatment. Cell angiogenesis: The cells treated with CuS NDs formed fitted junctions, mesh circles, branch nodes, and parallel cell lines. In vivo: Antibacterial activity: After CuS NDs+NIR treatment, the treated area became smaller. Wound healing: On day 12 after CuS NDs+NIR treatment, the wounds almost disappeared completely. |
| [44] | CN | HaCaT and HCEPC cells were used. Female BALB/c mice were used. | In vitro: The antibacterial activity of AuAgCu2O NSs with a laser (or without a laser) was evaluated on Escherichia coli and Staphylococcus aureus. In vivo: Mice were distributed into the following groups: control group, Ag NPs hydrogel PTT group, AuAgCu2O NSs hydrogel group, and AuAgCu2O NSs hydrogel PTT group. For the skin infection, Staphylococcus aureus suspension was used. | In vitro: Antibacterial activity, the bacterial integrity disruption, antibiofilm activity, and the behavior of AuAgCu2O NSs. In vivo: Wound healing and toxicity evaluation. | To develop a nanoagent to improve healing and antibacterial effects. | The hollow AuAg NSs were first synthesized using the well-used Ag NP templated galvanic replacement procedure. The hollow AuAg colloids were then added to an aqueous solution of PVP and Cu(NO3)2. After 30 min of stirring, N2H4·3H2O solution was immediately introduced into the solution. The olive-green hollow AuAgCu2O NSs were then centrifuged and cleaned three times before being redisposed in water for subsequent usage. To create a homogenous composite gel, sodium hyaluronate powders were progressively introduced into the hollow AuAgCu2O dispersion solution at a sufficient concentration while stirring. | In vitro: Antibacterial Activity: The reproduction of Escherichia coli and Staphylococcus aureus was inhibited after AuAgCu2O NSs with laser (or without laser). Bacterial Integrity Disruption: The death of Escherichia coli was almost complete after AuAgCu2O NSs treatment with laser. Antibiofilm Activity: AuAgCu2O plus laser showed a rare signal of biofilm activity. Behavior of AuAgCu2O NSs: In AuAgCu2O NSs treatment with laser, the highest migration rate was observed. In vivo: Wound healing: On day 8, the trauma area was smaller and an increase in the vessel number after AuAgCu2O NSs treatment plus laser was observed. Toxicity Evaluation: AuAgCu2O NSs-treated RBC did not show a broken form. |
| [45] | CN | NIH-313 cells and Sprague-Dawley rats were used. | In vitro: Antibacterial activity and determination of reactive oxygen species: Escherichia coli and Staphylococcus aureus were used for the antibacterial experiments. Cell viability and proliferation: NIH-3T3 cells were used to evaluate the activity of the hydrogel with or without Cu NPs cuts. qPCR analysis: The mRNA expressions of IL-1β, IL- 6, TNF-α, and IL-10 was evaluated. In vivo: Sprague-Dawley rats were used to evaluate the effects of hydrogel, hydrogel treatment plus laser, Cu NP-embedded hydrogel, and Cu NP-embedded hydrogel treatment plus laser. | In vitro: Antibacterial activity, determination of reactive oxygen species, cell viability and proliferation, and quantitative real-time PCR analysis. In vivo: Wound healing and immunofluorescence staining. | To design GelMA hydrogels combined with BACA/Cu NPs to promote wound healing and antibacterial activities against Gram-positive/negative bacteria. | Cu NPs were created in a modified version by reacting Cu ions with Na3C6H5O7. CuSO4·5H2O was then vigorously agitated in ultrapure water for 2 h. Following complete dissolution, a glass container was filled with ethylene glycol and distilled water, and the pH was adjusted to 10 using a strong NH3 solution. The reducing agent, Na3C6H5O7 solution, was then added to the previously described combination. After that, the vial was submerged in an oil bath at 90 °C until the blue transparent solution changed to the distinctive brownish-red hue when heated. Cu NPs were cleaned three times with ultrapure water before being stored in an ethanol solution at 20 °C. | In vitro: Antibacterial activity: Cu NP-embedded hydrogels increased Cu release after NIR laser irradiation. Determination of reactive oxygen species: No differences in lipid peroxidation were found. Cell viability and proliferation: All the hydrogel + CuNPs and hydrogel + CuNPs+NIR groups showed a slight decrease in the ability to proliferate NIH-3T3 cells. qPCR analysis: No differences in IL-1β, IL-6, TNF-α, and IL-10 expression were found. In vivo: Wound healing: The wound healing was faster in the Gel-MA/BACA-Cu NPs composite hydrogels treatment plus laser irradiation. |
| [46] | IN | L929 cells and Wistar rats were used. | In vitro: Cytocompatibility studies: L929 cell line was used to MTT assay according to the following treatments: gelatin, gelatin + GAGs + asiatic acid, gelatin + ZnO + CuO, and developed hydrogel composite. DNA quantification: Lysis buffer was used to cell lysate on day 1 and day 3. In vivo: Wound healing: Wistar rats were used to evaluate the efficacy of the wound dressing. Evaluation of TNF-α and MMP-2: Quantification of TNF-α and MMP-2 was realized on 7th, 14th, and 28th days after damage. | In vitro: Cytocompatibility studies and DNA quantification. In vivo: Wound healing and TNF-α and MMP-2 quantification. | To develop a hydrogel platform composed of biopolymer gelatin and glycosaminoglycans combined with asiatic acid, ZnO, and CuO NPs. To evaluate the efficacy of the wound dressings in burn wounds. | Dissolving gelatin in distilled water yielded a gelatin solution. After complete dissolution, appropriate amounts of (C14H21NO11)n, C13H21NO15S, C30H48O5, ZnO, and CuO NPs were added sequentially and mixed for 6 h. The solution was collected on a Petri plate after homogenization and lyophilized overnight. The hydrogels were then cross-linked using EDC coupling [47], washed with distilled water, and kept in a refrigerator at 20 °C until use. | In vitro: Cytocompatibility studies: From day 1 to day 3, an increase in cell numbers was observed in GAGs and the asiatic acid hydrogel group. However, a slow proliferation in cell number was observed in ZnO and CuO NP scaffolds. DNA quantification: From day 1 to day 3, an increased L929 cell number was found in the gelatin + GAGs + asiatic group. In vivo: Wound healing: On day 28, in the hydrogel composite group, a complete healing was observed. Evaluation of TNF-α and MMP-2: On day 7, a lower level of TNF-α was found in the hydrogel composite group in comparison with control. On day 7, MMP-2 levels were higher in the hydrogel than control group. |
| [48] | CN | HEK, HUVEC cells, and type 1 diabetic mice were used. | In vitro: SEM images: Bacterial suspensions in the presence of HvCuO@GOx or PBS with different concentrations of glucose were performed separately at 37 °C under 180 rpm. Scratch assay and endothelial tubule formation: HEK cells were incubated in the presence of HvCuO@GOx, HvCuO, or PBS. All groups were treated with glucose for 2 h. In vivo: Diabetes was induced by an intraperitoneal injection of streptozotocin in mice. Staphylococcus aureus-infected wounds were divided into the following treatments: hydrogel, HSHvCuO, and HSHvCuO@GOx. | In vitro: SEM images, cytotoxicity assay, scratch assay, and endothelial tubule formation. In vivo: Wound healing and treatment, and imaging in vivo. | To design a thermal-responsive spray for the synergistic restoration of DFU using its angiogenesis and antibacterial properties. | Virus-like silica nanoparticles and Cu(NO3)3·6H2O were dissolved in deionized water and agitated for 30 min. Then, (CH2)6N4 was added, and the aforementioned mixture was constantly agitated for 2 h. Final samples were centrifuged multiple times with deionized water to eliminate unreacted residues, then dried in an oven. Finally, the virus-like mesoporous silica template was etched with 0.1 M Na2CO3, agitated, and washed three times with deionized water. HvCuO were produced after drying in an oven overnight. | In vitro: SEM images: In the NPs group, the rough surface was nicely maintained. No changes were found in the morphology of HvCuO@GOx nanoshells even without adding glucose for 12 h. After 2 h, the glucose concentration was dramatically reduced in the HvCuO@GOx presence in comparison with HvCuO. The most effective bactericidal action was found in HSHvCuO@GOx dressing. Cytotoxicity assay: A negligible cytotoxicity was found in HEK and HUVEC cells treated with different HvCuO@GOx concentrations. Scratch assay: A significant cell migration was found in HEK cells treated with HvCuO@GOx. Endothelial tubule formation: A higher number of tubule junctions was found in HUVEC cells treated with HvCuO@GOx at 150 μg/mL concentration. In vivo: Wound healing and treatment: At day 15, the wound healing was almost completely healed in the HSHvCuO@GOx group. Imaging in vivo: The highest quantity of CD34-positive cells was found in the HSHvCuO@GOx group. |
| [49] | CN | Recombinant human bFGF, NIH-313 cells, and HUVEC cells. | In vitro: Mechanical properties: A universal machine tester was used to evaluate the mechanical properties of the compounds. Antibacterial activity: The antibacterial ability of compounds was assessed using Escherichia coli and Staphylococcus aureus. Cell cytotoxicity and proliferation assessment and migration and tubule formation activities: Different ionic extractions were used to culture NIH/3T3 and HUVEC cells. In vivo: Rats were used to prepare the wound model. Each one was treated with GelMA, 5% Cu-NA@GelMA, or 5% Cu-NA-bFGF@GelMA. The skin was extracted for further histological and immunohistochemical studies on days 3, 7, and 14. | In vitro: Mechanical properties, antibacterial activity, cell cytotoxicity and proliferation assessment, and migration and tubule formation activities. In vivo: Wound healing and histopathologic evaluation. | To prepare a new Cu-nicotinic acid based on using biomolecules of nicotinic acid. | C2H5NO2 and Cu2(OAc)4(H2O)2 were mixed in deionized water and CH2OH solutions in various ratios, then heated and agitated. Cu2(OAc)4(H2O)2 solution was then swiftly added to the C2H5NO2 solution while rapidly swirling at 1000 rpm. After stirring, the solution was centrifuged, and then washed with deionized water and ethanol to get the copper-nicotinic acid. Finally, the blue powders were obtained by the freeze-drying technique and kept at 4 °C for future study. | In vitro: Mechanical properties: the best elasticity was found in the CuNA@GelMAs group. Antibacterial activity: CuNA@GelMAs showed good antibacterial ability toward E. coli and S. aureus. Moreover, an enhancement in antibacterial properties was observed after increasing the CuNA content in the hydrogels. Cell cytotoxicity and proliferation assessment: CuNA-bFGF@GelMA, CuNA@GelMA, and GelMA have shown no significant difference. Migration and tubule formation activities: In HUVEC and NIH/3T3 cells, increased migration and total segment length in the GelMA group were found. In vivo: Wound healing: In CuNA@GelMA and Cu-NA-bFGF@ GelMA treatments, a decreased percentage of wound closures was found in comparison to other groups. Histopathologic evaluation: More new blood vessels, regular epithelium, and mild inflammatory responses in CuNA@GelMA and CuNA-bFGF@GelMA treatments were found. |
| [50] | USA | Immortalized HEKas and HDF cells. Diabetic (db/db) mice were used. | In vitro: Cytotoxicity and apoptosis assays: Different concentrations of PPCN, H3BTC, CuSO4, H-CuSO4, HKUST-1 NPs, or H-HKUST-1 were used as treatments in HEKa and HDF cells. Cell migration assay: HEKa and HDF cells were used in a confluent monolayer. In vivo: Mice were separated into the following groups: PBS-treated, HKUST-1-treated, PPCN-treated, and H-HKUST-1-treated. | In vitro: Cytotoxicity, apoptosis, and scratch assays. In vivo: Wound healing and histopathological analysis. | To assess whether HKUST-1 NPs embedded within an antioxidant thermoresponsive citrate-based hydrogel would decrease Cu cytotoxicity and accelerate the healing process in a diabetic model. | As previously reported, the author’s group synthesized PPCN [51]. First, a PPCac was made by polycondensing C2H8O7, PEG, and C9H12O5. PPCac was then reacted with repurified NIPAM overnight by free radical polymerization using AIBN as the free radical initiator. Precipitation and purification with (C2H5)2O yielded the reaction product, PPCN. The PPCN was then dissolved in PBS, neutralized with NaOH to pH 7.4, and stored as a lyophilized powder for further use. HKUST-1 NPs were created using a previously described process (Xiao et al., 2013). To make a gel solution, Cu(CO2CH3)2 dissolved in distilled water was dropwise added to H3BTC diluted in ethanol, followed by stirring for 20 min. To get pure HKUST-1, the suspension was centrifuged, and the precipitate was washed with an ethanol/water solution. H-HKUST-1 was created by adding HKUST-1 NPs to a PPCN solution with Cu at room temperature. | In vitro: Cytotoxicity assay: A lower toxicity inH-HKUST-1 treatment was found (1 × 10−3 M). Apoptosis assay: Cell apoptosis was 10.7 ± 2.5% and 17.0 ± 5.4% in HEKa and HDF cells after H-HKUST-1 treatment. Cell migration assay: The highest cell migration after H-HKUST-1 treatment in HEKa and HDF cells was found. In vivo: Wound healing: On day 21, the wound almost completely healed after H-HKUST-1 treatment. However, PBS, PPCN, and HKUST-1 NP groups healed at days 39, 39, and 37, respectively. A faster healing time in H-HKUST-1 treatment in comparison to PBS, PPCN, and HKUST-1 NP groups was found. Histopathological analysis: A more stable and densely perfused vascular network in H-HKUST-1 and HKUST-1 NP treatment in comparison to PBS and PPCN groups was found. In the HKUST-1 and H-HKUST-1 groups, an increased blood vessel number and area, as well as neovascularization, were found. Higher granulation tissue and blood vessel numbers in HKUST-1- and H-HKUST-1-treated wounds were found, respectively. Smaller granulation tissue in the H-HKUST-1-treated wound was found. |
| [52] | USA | Immortalized HEKa, HDF, and HUVEC cells. Diabetic (db/db) and non-diabetic (C57BL/6) mice were used. | In vitro: Cytotoxicity and scratch assay: Folic acid, HKUST-1, and F-HKUST-1 were used as treatments in HEKa and HDF cells. Endothelial tubule formation assay: PBS, folic acid, HKUST-1, and F-HKUST-1 were used as treatments in HUVEC cells. In vivo: Three treatments (HKUST-1, F-HKUST-1, and folic acid) were used to evaluate wound healing at different time points. | In vitro: Cytotoxicity, scratch, and endothelial tubule formation assays. In vivo: Wound healing and histopathology analysis. | To evaluate the modification of HKUST-1, to release Cu2+, reducing cytotoxicity and improving wound healing rates. | HKUST-1 was synthesized in the manner previously reported (17, 46). Following the modification HKUST-1 synthesis technique to include C19H19N7O6 created F-HKUST-1. C19H19N7O6 in DMSO was combined with H3BTC in ethanol. The H3BTC/C19H19N7O6 solution was then treated with ethanol. To generate a green, gel-like suspension, Cu(CO2CH3)2 dissolved in deionized water was added dropwise to the H3BTC/C19H19N7O6 solution and agitated at room temperature. To get pure F-HKUST-1, the suspensions were centrifuged and the precipitates were washed with reaction media DMSO, ethanol, or water, respectively. Purified particles were kept at −80 °C in ethanol. | In vitro: Cytotoxicity assay: A lower toxicity in F-HKUST-1 treatment was found (0.5 mM). Scratch Assay: The highest migration rate in F-HKUST-1-treated cells was found. Endothelial tubule formation assay: The largest number of tubule junctions in F-HKUST-1-treated HUVEC cells was found. In vivo: Wound healing: At days 19, 21, and 30 post-wounding, an improved wound healing in the F-HKUST-1 group was found (p < 0.05). Histopathology analysis: At day 30, a 107.8 ± 18.1 μm granulation tissue thickness in the F-HKUST-1 group was found. |
| [53] | CN | HUVEC cells and female BALB/c mice were used. | In vitro: Antibacterial performance: For the optimal concentration, nanoliquid dressings at different concentrations were used (CuS nanoplates and HNO3). Anti-biofilm assay: Crystal violet assay was used. In vivo: Wound healing: Mice were separated into the following groups: control; HNO3; CuS; CuS + HNO3; CuS + NIR; and CuS + HNO3+NIR. | In vitro: Antibacterial performance and anti-biofilm assay. In vivo: Wound healing. | To design a novel nanoliquid dressing based on a mild photothermal heating strategy to provide safe healing of biofilm-infected wounds. | A container was filled with sulfur powder and 1-ODE. After the oxygen was removed, the mixture was heated. Then, the sulfur powder was dissolved and insulated for future use. In another container, there was CuCl2 powder, OM, and 1-ODE. When the CuCl2-containing mixture was heated in a vacuum, a brilliant yellow solution was produced. The container was then quickly injected into a sulfur-containing 1-ODE solution. In between injection cycles, CuS nanocrystals were cultured. After six injection sessions, CuS nanoplates were created. The generated CuS nanoplates were precipitated and centrifuged using an excess of ethanol after the reaction solution was cooled to room temperature. The precipitate was washed and kept at room temperature in chloroform for future use. | In vitro: Antibacterial performance: An ~80% cell viability in CuS-CTAB nanoplate-treated or HNO3-treated HUVEC cells was found. Anti-biofilm assay: A thickness reduction of biofilms in the CuS−HNO3 + NIR group was found in comparison to untreated biofilm. In vivo: Wound healing: At day 15, 72.9% and 98.6% of wound closure in normal saline and CuS−HNO3 + NIR-treated mice were found. |
| Measurement | Wound Changes | Evaluation Method | References |
|---|---|---|---|
| Cytotoxicity | Cytotoxicity of Cu-MBG was well-tolerated (at 0.1 and 1 mg/mL). | Metabolic assay PrestoBlueTM | [42] |
| The generation of hydroxyl radicals in the biofilm environment may be of insignificant toxicity in diabetic wounds. | Endothelial tubule formation | [48] | |
| Good in vivo safety and biocompatibility have been suggested after CuS NDs + NIR treatment, where no histological changes or toxicity within the treatment period were found. | H&E staining | [43] | |
| After treatment with HKUST-1 and F-HKUST-1, HEKas and HDF cells exhibited enhanced migration. In addition, the highest cell migration in F-HKUST-1 has been found. Its enhanced migration is due to the Cu2+ presence in HKUST-1 and F-HKUST-1 groups. | Scratch assay | [52] | |
| About NIH-3T3 cells, no significant in vitro cytotoxicity has been described. | CCK-8 assay | [45] | |
| On day 28, no differences in the inflammatory cells were found. | Haematological analysis | [46] | |
| The use of hydrogel + NPs treatment in second-degree burns is safe because no changes in markers of liver and kidney have been found. | Biochemistry analysis | [46] | |
| No significant cytotoxicity after treatment with different concentration of USNPs was found. Good biocompatibility and normal cytoskeleton morphology in HEK293 cells treated with USNPs (200 ng mL−1) have been described. | CCK-8 assay | [41] | |
| After 30 days, no cardiovascular damage after USNP injection has been found. | Hemolysis assay | [41] | |
| After 24 h, no tissue damage or inflammatory lesions in the genitourinary system after USNPs injection have been described. | Hemolysis assay | [41] | |
| A significant inhibition in the MAPK pathway after USNPs treatment was found, showing it might decrease renal injury by decreasing the ROS level. | Principal component analysis | [41] | |
| As the incubation period wore on, the number of cells grew for all groups. The viability of cells decreased for the BC/Cu60- and BC/Cu100-treated groups (46 ± 6% and 30 ± 8%, respectively). After BC/Cu20 treatments, NHDF cells did not show decreased cell viability in comparison to the control group; after BC/Cu60 and BC/Cu100 treatments, NHDF cells showed decreased cell viability. | CCK-8 assay and calcein staining | [40] | |
| Antibacterial response | Antibacterial effects of Cu-MBG (100 μg/mL) against Pseudomonas aeruginosa and Staphylococcus aureus with 1.2–3.5 log reductions were found. In addition, a reduced existing biofilm after Cu-MBG treatment was described. | Brain heart infusion | [42] |
| Eradication of bacteria after CuS NDs (45 μg/mL) treatment was found. Likewise, a higher antibacterial effect in the CuS NDs+NIR group than in the CuS NP + NIR group was found. | Growth-inhibition assay | [43] | |
| In bacteria after CuS NPs+NIR and CuS NDs treatments, outer membranes were damaged. However, in bacterial cell walls, a loss of integrity after CuS NDs+NIR treatment was found. In addition, after CuS NDs+NIR treatment, the cytoplasm displayed aggregates in Escherichia coli and Staphylococcus aureus, confirming the cell damage. | TEM | [43] | |
| After incubation with AuAgCu2O NSs, several dead cells were detected in the Escherichia coli incubation; however, almost complete death of bacteria after AuAgCu2O NSs treatment with a laser was found. | SYTO9/PI live/dead fluorescent staining assay | [44] | |
| After 6 and 24 h of incubation, effective antibacterial effects in GelMA/BACA-CuNPs hydrogels+NIR against Escherichia coli and Staphylococcus aureus have been described. | In vitro antibacterial assay | [45] | |
| The inhibition zone for Escherichia coli and Staphylococcus aureus was 3.1 ± 0.8 mm and 2.6 ± 0.3 mm for the hydrogel; it was 5.3 ± 0.2 mm and 4.9 ± 0.6 mm for the gelatin + ZnO group, whereas the inhibition zone was 4.8 ± 0.7 mm and 3.8 ± 0.3 mm for the gelatin + CuO treatment. | Disc diffusion method | [46] | |
| BC/Cu membranes showed a higher inhibition zone against Staphylococcus aureus for up to 90 days. | Disk diffusion method | [40] | |
| A slow release of Cu in membranes was found even at 90 days, and remaining membranes with Cu were found. However, a faster release of Cu was found when a 37 °C incubator was used; after 24 h, the release of Cu of membranes was almost complete. | Disk diffusion method | [40] | |
| Wound healing | At 24 h, the outgrowth of endothelial cells started for most aortic rings, and the outgrowth area increased from 0.3 to 1.5 mm2 when VEGF was added. | Aortic ring assay | [42] |
| Increases in the number of junctions and total vessel length in MBG and Cu-MBG groups were found. | CAM assay | [42] | |
| The vascular network formation was promoted by H-HKUST-1 and HKUST-1 NP treatments. In addition, blood vessel area, blood vessel number, and neovascularization in HKUST-1-and H-HKUST-1 were increased. | OCTA | [50] | |
| The highest functional levels of blood vessel oxygen of the HSHvCuO@GOx group were found, confirming the properties of HSHvCuO@GOx in hypoxia alleviation. | Photoacoustic imaging in vivo | [48] | |
| A total of 14 days was necessary for the F-HKUST-1 group to close 50% of the wound area, whereas a total of 19 days was necessary for the other groups. | Dermal excision wound model | [52] | |
| Tight connections, parallel cell lines, and mesh circles in AuAgCu2O NSs treatment groups with or without laser irradiation indicated a late phase of angiogenesis. | Matrigel assay | [44] | |
| Effective antibacterial capacity in hydrogel + CuNPs + NIR after CD86+ and CD206 intensity analysis was found. | IHC | [45] | |
| A larger degradation rate in Cu NP hydrogels than in simple hydrogels was found. | Masson’s trichrome and H&E staining | [45] | |
| On day 7, decreased inflammation and high tissue remodeling as a consequence of low levels of TNF-α and high levels of MMP-2 in the hydrogel composite were found. | ELISA kit | [46] | |
| A faster healing rate in the USNPs group in comparison to the control group was found during days 4, 7, 9, and 15 post-surgery (p < 0.01). | H&E staining | [41] |
| References | Study Design | Population | Method of Allocation to Intervention (or Comparison) | Outcomes | Analyses | Summary | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | ||
| [40] | Clinical trial | ++ | ++ | + | ++ | ++ | NR | NA | ++ | ++ | + | ++ | NA | NA | ++ | ++ | ++ | ++ | + | ++ | ++ | NR | + | ++ | ++ | + | ++ | + |
| [41] | Clinical trial | ++ | + | + | + | ++ | NR | NR | + | + | NR | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | + | ++ | ++ | ++ | ++ | + |
| [42] | Clinical trial | ++ | + | + | NR | ++ | NR | NR | + | + | NA | NA | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | NR | NR | + | + | ++ | ++ | ++ | ++ |
| [43] | Clinical trial | ++ | + | + | NR | ++ | NR | NR | ++ | ++ | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | ++ | ++ | ++ | ++ | ++ | ++ |
| [44] | Clinical trial | ++ | + | + | NR | ++ | NR | NR | ++ | ++ | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | + | NR | + | ++ | ++ | ++ | ++ | ++ |
| [45] | Clinical trial | ++ | + | + | NR | ++ | NR | NR | ++ | ++ | ++ | + | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | ++ | ++ | ++ | ++ | ++ | ++ |
| [46] | Experimental study | ++ | + | + | ++ | ++ | NR | NA | ++ | ++ | + | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | ++ | ++ | ++ | ++ | ++ | ++ |
| [48] | Clinical trial | ++ | + | + | NR | ++ | NR | NR | ++ | ++ | NR | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | NR | NR | ++ | + | ++ | ++ | ++ | ++ |
| [49] | Experimental study | + | + | + | + | ++ | NR | NA | ++ | + | + | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | NR | NR | ++ | + | ++ | ++ | ++ | ++ |
| [50] | Clinical trial | ++ | + | + | ++ | ++ | NR | NR | ++ | ++ | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | ++ | NR | + | + | ++ | ++ | ++ | ++ |
| [52] | Clinical trial | ++ | + | + | NR | + | NR | NR | ++ | ++ | + | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | + | NR | + | ++ | ++ | ++ | ++ | ++ |
| [53] | Clinical trial | ++ | + | + | ++ | ++ | NR | NA | ++ | ++ | ++ | ++ | NA | NA | ++ | ++ | ++ | ++ | ++ | ++ | + | NR | ++ | + | ++ | + | ++ | ++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval, C.; Ríos, G.; Sepúlveda, N.; Salvo, J.; Souza-Mello, V.; Farías, J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics 2022, 14, 1838. https://doi.org/10.3390/pharmaceutics14091838
Sandoval C, Ríos G, Sepúlveda N, Salvo J, Souza-Mello V, Farías J. Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review. Pharmaceutics. 2022; 14(9):1838. https://doi.org/10.3390/pharmaceutics14091838
Chicago/Turabian StyleSandoval, Cristian, Gemima Ríos, Natalia Sepúlveda, Jessica Salvo, Vanessa Souza-Mello, and Jorge Farías. 2022. "Effectiveness of Copper Nanoparticles in Wound Healing Process Using In Vivo and In Vitro Studies: A Systematic Review" Pharmaceutics 14, no. 9: 1838. https://doi.org/10.3390/pharmaceutics14091838