Systematic Review of the Roost-Site Characteristics of North American Forest Bats: Implications for Conservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Region and Species
2.2. Data Sources and Search Strategy
2.3. Systematic Review
2.4. Assessment of Potential Umbrella Species
3. Results
3.1. Search Results
3.2. Summary of Roost-Site Characteristics
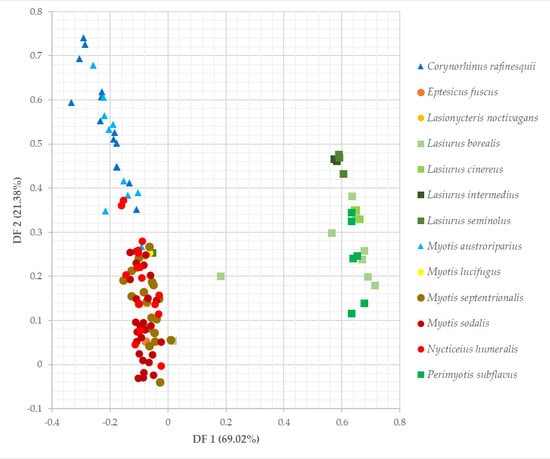
3.3. Niche Overlap of Eastern Temperate Forest Bats
3.4. Spatial Overlap Among Eastern Temperate Forest Bats
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Species Pair | DBH | T.Ht | R.Ht | CC | Decid | Snag | Fol |
|---|---|---|---|---|---|---|---|
| Eptesicus fuscus - Corynorhinus rafinesquii | <0.0001 | 0.9639 | 0.9999 | 1.0000 | 0.4085 | 0.6792 | 1.0000 |
| Lasionycteris noctivagans - Corynorhinus rafinesquii | 0.0045 | 0.9934 | 1.0000 | 0.9644 | 0.1918 | 0.9999 | 1.0000 |
| Lasiurus borealis - Corynorhinus rafinesquii | <0.0001 | 0.9592 | 0.4567 | 1.0000 | 0.9999 | 0.9714 | <0.0001 |
| Lasiurus cinereus - Corynorhinus rafinesquii | <0.0001 | 0.9586 | 0.0020 | 1.0000 | 0.1050 | 0.9934 | <0.0001 |
| Lasiurus intermedius - Corynorhinus rafinesquii | 0.0498 | 0.9900 | 0.9992 | 0.9479 | 1.0000 | 0.9983 | <0.0001 |
| Lasiurus seminolus - Corynorhinus rafinesquii | <0.0001 | 0.8938 | 0.0838 | 1.0000 | <0.0001 | 0.9983 | <0.0001 |
| Myotis austroriparius - Corynorhinus rafinesquii | 0.1487 | 1.0000 | 0.9256 | 0.9966 | 1.0000 | 1.0000 | 1.0000 |
| Myotis lucifugus - Corynorhinus rafinesquii | 0.0007 | 0.5812 | 0.9989 | 1.0000 | 1.0000 | 0.7967 | 1.0000 |
| Myotis septentrionalis - Corynorhinus rafinesquii | <0.0001 | 0.9305 | 0.5664 | 1.0000 | 0.9903 | 0.1896 | 1.0000 |
| Myotis sodalis - Corynorhinus rafinesquii | <0.0001 | 1.0000 | 0.0723 | 0.7962 | 0.7977 | 0.0080 | 1.0000 |
| Nycticeius humeralis - Corynorhinus rafinesquii | <0.0001 | 0.8844 | 0.0749 | 1.0000 | 0.1694 | 0.6818 | 1.0000 |
| Perimyotis subflavus - Corynorhinus rafinesquii | <0.0001 | 1.0000 | 0.0572 | 0.9970 | 1.0000 | 1.0000 | <0.0001 |
| Lasionycteris noctivagans - Eptesicus fuscus | 1.0000 | 0.7767 | 1.0000 | 0.9999 | 0.9995 | 0.7167 | 1.0000 |
| Lasiurus borealis - Eptesicus fuscus | 1.0000 | 1.0000 | 0.9990 | 0.9994 | 0.7165 | 0.1480 | <0.0001 |
| Lasiurus cinereus - Eptesicus fuscus | 1.0000 | 0.5247 | 0.1584 | 0.9999 | 1.0000 | 0.2682 | <0.0001 |
| Lasiurus intermedius - Eptesicus fuscus | 1.0000 | 1.0000 | 0.9879 | 1.0000 | 0.8063 | 0.4065 | <0.0001 |
| Lasiurus seminolus - Eptesicus fuscus | 1.0000 | 0.4137 | 0.7530 | 1.0000 | 0.8792 | 0.4065 | <0.0001 |
| Myotis austroriparius - Eptesicus fuscus | 0.0107 | 0.9227 | 0.9401 | 0.9892 | 0.4740 | 0.7647 | 1.0000 |
| Myotis lucifugus - Eptesicus fuscus | 0.9999 | 0.9985 | 1.0000 | 1.0000 | 0.9456 | 1.0000 | 1.0000 |
| Myotis septentrionalis - Eptesicus fuscus | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.7836 | 1.0000 | 1.0000 |
| Myotis sodalis - Eptesicus fuscus | 0.9994 | 0.9933 | 0.9998 | 1.0000 | 0.8953 | 1.0000 | 1.0000 |
| Nycticeius humeralis - Eptesicus fuscus | 0.9901 | 1.0000 | 0.9968 | 1.0000 | 0.9982 | 0.9990 | 1.0000 |
| Perimyotis subflavus - Eptesicus fuscus | 1.0000 | 0.9799 | 0.9081 | 1.0000 | 0.6081 | 0.4708 | <0.0001 |
| Lasiurus borealis - Lasionycteris noctivagans | 1.0000 | 0.8224 | 0.9126 | 0.8210 | 0.3614 | 1.0000 | <0.0001 |
| Lasiurus cinereus - Lasionycteris noctivagans | 1.0000 | 1.0000 | 0.0420 | 0.9642 | 0.9997 | 1.0000 | <0.0001 |
| Lasiurus intermedius - Lasionycteris noctivagans | 1.0000 | 0.8509 | 1.0000 | 1.0000 | 0.4353 | 1.0000 | <0.0001 |
| Lasiurus seminolus - Lasionycteris noctivagans | 1.0000 | 1.0000 | 0.3775 | 0.9861 | 1.0000 | 1.0000 | <0.0001 |
| Myotis austroriparius - Lasionycteris noctivagans | 0.1904 | 0.9993 | 0.9999 | 0.6966 | 0.2190 | 0.9999 | 1.0000 |
| Myotis lucifugus - Lasionycteris noctivagans | 1.0000 | 0.3774 | 0.9999 | 0.9999 | 0.6629 | 0.7299 | 1.0000 |
| Myotis septentrionalis - Lasionycteris noctivagans | 1.0000 | 0.8201 | 0.9953 | 0.9933 | 0.4148 | 0.6841 | 1.0000 |
| Myotis sodalis - Lasionycteris noctivagans | 1.0000 | 0.9661 | 0.9172 | 1.0000 | 0.5285 | 0.3975 | 1.0000 |
| Nycticeius humeralis - Lasionycteris noctivagans | 0.9996 | 0.7824 | 0.8250 | 0.9927 | 0.8419 | 0.8877 | 1.0000 |
| Perimyotis subflavus - Lasionycteris noctivagans | 1.0000 | 0.9950 | 0.5225 | 1.0000 | 0.2883 | 1.0000 | <0.0001 |
| Lasiurus cinereus - Lasiurus borealis | 1.0000 | 0.4030 | 0.3624 | 1.0000 | 0.3441 | 1.0000 | 0.8146 |
| Lasiurus intermedius - Lasiurus borealis | 0.9985 | 1.0000 | 0.4986 | 0.6826 | 1.0000 | 1.0000 | 0.9299 |
| Lasiurus seminolus - Lasiurus borealis | 0.9913 | 0.2325 | 0.9780 | 1.0000 | 0.0005 | 1.0000 | 0.8146 |
| Myotis austroriparius - Lasiurus borealis | <0.0001 | 0.9141 | 0.0794 | 1.0000 | 1.0000 | 0.9783 | <0.0001 |
| Myotis lucifugus - Lasiurus borealis | 0.9843 | 0.9510 | 0.9999 | 0.9997 | 1.0000 | 0.3353 | <0.0001 |
| Myotis septentrionalis - Lasiurus borealis | 0.9560 | 1.0000 | 0.9970 | 0.9217 | 1.0000 | 0.0030 | <0.0001 |
| Myotis sodalis - Lasiurus borealis | 0.4913 | 0.9968 | 1.0000 | 0.2186 | 0.9995 | <0.0001 | <0.0001 |
| Nycticeius humeralis - Lasiurus borealis | 0.2946 | 1.0000 | 1.0000 | 0.9863 | 0.7580 | 0.0325 | <0.0001 |
| Perimyotis subflavus - Lasiurus borealis | 1.0000 | 0.9886 | 0.9996 | 0.8858 | 1.0000 | 0.9996 | 0.4902 |
| Lasiurus intermedius - Lasiurus cinereus | 1.0000 | 0.7512 | 0.0082 | 0.9672 | 0.6015 | 1.0000 | 1.0000 |
| Lasiurus seminolus - Lasiurus cinereus | 1.0000 | 1.0000 | 0.9960 | 1.0000 | 0.8563 | 1.0000 | 1.0000 |
| Myotis austroriparius - Lasiurus cinereus | 0.0014 | 0.9977 | 0.0003 | 1.0000 | 0.1574 | 0.9939 | <0.0001 |
| Myotis lucifugus - Lasiurus cinereus | 0.9998 | 0.1966 | 0.2150 | 1.0000 | 0.8771 | 0.4231 | <0.0001 |
| Myotis septentrionalis - Lasiurus cinereus | 1.0000 | 0.3362 | 0.0298 | 0.9998 | 0.3951 | 0.0580 | <0.0001 |
| Myotis sodalis - Lasiurus cinereus | 0.9984 | 0.7724 | 0.0921 | 0.9511 | 0.5683 | 0.0055 | <0.0001 |
| Nycticeius humeralis - Lasiurus cinereus | 0.9767 | 0.2887 | 0.2215 | 1.0000 | 0.9702 | 0.2341 | <0.0001 |
| Perimyotis subflavus - Lasiurus cinereus | 1.0000 | 0.9779 | 0.7974 | 0.9968 | 0.2726 | 0.9999 | 1.0000 |
| Lasiurus seminolus - Lasiurus intermedius | 1.0000 | 0.6900 | 0.1160 | 0.9867 | 0.0159 | 1.0000 | 1.0000 |
| Myotis austroriparius - Lasiurus intermedius | 0.6534 | 0.9741 | 1.0000 | 0.5852 | 1.0000 | 0.9982 | <0.0001 |
| Myotis lucifugus - Lasiurus intermedius | 1.0000 | 0.9999 | 0.9691 | 1.0000 | 1.0000 | 0.5183 | <0.0001 |
| Myotis septentrionalis - Lasiurus intermedius | 1.0000 | 1.0000 | 0.7525 | 0.9910 | 1.0000 | 0.2092 | <0.0001 |
| Myotis sodalis - Lasiurus intermedius | 1.0000 | 0.9982 | 0.4294 | 1.0000 | 0.9996 | 0.0462 | <0.0001 |
| Nycticeius humeralis - Lasiurus intermedius | 1.0000 | 1.0000 | 0.3237 | 0.9915 | 0.9593 | 0.5000 | <0.0001 |
| Perimyotis subflavus - Lasiurus intermedius | 0.9977 | 0.9932 | 0.1537 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Myotis austroriparius - Lasiurus seminolus | 0.0033 | 0.9924 | 0.0146 | 0.9986 | 0.0001 | 0.9982 | <0.0001 |
| Myotis lucifugus - Lasiurus seminolus | 1.0000 | 0.1497 | 0.8376 | 1.0000 | 0.1451 | 0.5183 | <0.0001 |
| Myotis septentrionalis - Lasiurus seminolus | 1.0000 | 0.1607 | 0.5201 | 1.0000 | 0.0004 | 0.2092 | <0.0001 |
| Myotis sodalis - Lasiurus seminolus | 1.0000 | 0.5736 | 0.8194 | 0.9683 | 0.0008 | 0.0462 | <0.0001 |
| Nycticeius humeralis - Lasiurus seminolus | 0.9999 | 0.1338 | 0.9540 | 1.0000 | 0.0201 | 0.5000 | <0.0001 |
| Perimyotis subflavus - Lasiurus seminolus | 0.9908 | 0.9457 | 1.0000 | 0.9997 | 0.0005 | 1.0000 | 1.0000 |
| Myotis lucifugus - Myotis austroriparius | 0.1775 | 0.5022 | 0.8698 | 0.9929 | 1.0000 | 0.8401 | 1.0000 |
| Myotis septentrionalis - Myotis austroriparius | <0.0001 | 0.8827 | 0.0486 | 0.8699 | 0.9976 | 0.4003 | 1.0000 |
| Myotis sodalis - Myotis austroriparius | <0.0001 | 0.9995 | 0.0034 | 0.3331 | 0.9307 | 0.0476 | 1.0000 |
| Nycticeius humeralis - Myotis austroriparius | 0.0004 | 0.8302 | 0.0047 | 0.9386 | 0.3696 | 0.8620 | 1.0000 |
| Perimyotis subflavus - Myotis austroriparius | <0.0001 | 1.0000 | 0.0060 | 0.7912 | 1.0000 | 1.0000 | <0.0001 |
| Myotis septentrionalis - Myotis lucifugus | 1.0000 | 0.9248 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Myotis sodalis - Myotis lucifugus | 1.0000 | 0.7098 | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 1.0000 |
| Nycticeius humeralis - Myotis lucifugus | 1.0000 | 0.9496 | 0.9996 | 1.0000 | 0.9972 | 0.9978 | 1.0000 |
| Perimyotis subflavus - Myotis lucifugus | 0.9820 | 0.6444 | 0.9581 | 1.0000 | 1.0000 | 0.6469 | <0.0001 |
| Myotis sodalis - Myotis septentrionalis | 0.9992 | 0.9936 | 0.9940 | 0.8859 | 1.0000 | 0.9929 | 1.0000 |
| Nycticeius humeralis - Myotis septentrionalis | 0.9566 | 1.0000 | 0.9463 | 1.0000 | 0.8000 | 0.9979 | 1.0000 |
| Perimyotis subflavus - Myotis septentrionalis | 0.9747 | 0.9852 | 0.6357 | 1.0000 | 0.9999 | 0.0697 | <0.0001 |
| Nycticeius humeralis - Myotis sodalis | 0.9999 | 0.9814 | 1.0000 | 0.9417 | 0.9622 | 0.4726 | 1.0000 |
| Perimyotis subflavus - Myotis sodalis | 0.7046 | 1.0000 | 0.9486 | 1.0000 | 0.9918 | 0.0023 | <0.0001 |
| Perimyotis subflavus - Nycticeius humeralis | 0.4754 | 0.9694 | 0.9980 | 1.0000 | 0.6663 | 0.3675 | <0.0001 |


References
- Kunz, T.H. Ecology of Bats; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- MammalDiversity.org. Mammal Diversity Database. Available online: www.mammaldiversity.org (accessed on 6 January 2020).
- Burgin, C.J.; Colella, J.P.; Kahn, P.L.; Upham, N.S. How many species of mammals are there? J. Mammal. 2018, 99, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.E.; Smith, S.E. Craseonycteris thonglongyai. Mammal. Species 1981, 1–4. [Google Scholar] [CrossRef]
- Ingle, N.R.; Heaney, L.R. A key to the bats of the Philippine Islands. Fieldiana Zool. 1992, 69, 1–44. [Google Scholar]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Maine, J.J.; Boyles, J.G. Bats initiate vital agroecological interactions in corn. Proc. Natl. Acad. Sci. USA 2015, 112, 12438–12443. [Google Scholar] [CrossRef] [Green Version]
- Maslo, B.; Valentin, R.; Leu, K.; Kerwin, K.; Hamilton, G.C.; Bevan, A.; Fefferman, N.H.; Fonseca, D.M. Chirosurveillance: The use of native bats to detect invasive agricultural pests. PLoS ONE 2017, 12, e0173321. [Google Scholar] [CrossRef]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Fleming, T.H.; Geiselman, C.; Kress, W.J. The evolution of bat pollination: A phylogenetic perspective. Ann. Bot. 2009, 104, 1017–1043. [Google Scholar] [CrossRef]
- Tremlett, C.J.; Moore, M.; Chapman, M.A.; Zamora-Gutierrez, V.; Peh, K.S.H. Pollination by bats enhances both quality and yield of a major cash crop in Mexico. J. Appl. Ecol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Mahandran, V.; Murugan, C.M.; Marimuthu, G.; Nathan, P.T. Seed dispersal of a tropical deciduous Mahua tree, Madhuca latifolia (Sapotaceae) exhibiting bat-fruit syndrome by pteropodid bats. Glob. Ecol. Conserv. Manag. Bats For. 2018, 14, e00396. [Google Scholar] [CrossRef]
- Saldaña-Vázquez, R.A.; Castaño, J.H.; Baldwin, J.; Pérez-Torres, J. Does seed ingestion by bats enhance germination? A new meta-analysis 15 years later. Mammal. Rev. 2019, 49, 201–209. [Google Scholar] [CrossRef]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.F. The impact of climate change on smallholder and subsistence agriculture. Proc. Natl. Acad. Sci. USA 2007, 104, 19680–19685. [Google Scholar] [CrossRef] [Green Version]
- Frick, W.F.; Kingston, T.; Flanders, J. A review of the major threats and challenges to global bat conservation. Ann. N. Y. Acad. Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Rhodes, J.R.; Watson, J.E.; Lefevre, J.; Atkinson, S.; Possingham, H.P. Use of surrogate species to cost-effectively prioritize conservation actions. Conserv. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Roberge, J.M.; Angelstam, P. Usefulness of the umbrella species concept as a conservation tool. Conserv. Biol. 2004, 18, 76–85. [Google Scholar] [CrossRef]
- Seddon, P.J.; Leech, T. Conservation short cut, or long and winding road? A critique of umbrella species criteria. Oryx 2008, 42, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Caro, T.; O’Doherty, G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Kingston, T. Research priorities for bat conservation in Southeast Asia: A consensus approach. Biodivers. Conserv. 2010, 19, 471–484. [Google Scholar] [CrossRef]
- Scanlon, A.T.; Petit, S. Capture success of Fijian bats (Pteropodidae) and their evaluation as umbrella species for conservation. Pac. Conserv. Biol. 2016, 21, 315–326. [Google Scholar] [CrossRef]
- Caro, T. Umbrella species: Critique and lessons from East Africa. Anim. Conserv. 2003, 6, 171–181. [Google Scholar] [CrossRef]
- Fleishman, E.; Murphy, D.D.; Brussard, P.F. A new method for selection of umbrella species for conservation planning. Ecol. Appl. 2000, 10, 569–579. [Google Scholar] [CrossRef]
- Favreau, J.M.; Drew, C.A.; Hess, G.R.; Rubino, M.J.; Koch, F.H.; Eschelbach, K.A. Recommendations for assessing the effectiveness of surrogate species approaches. Biodivers. Conserv. 2006, 15, 3949–3969. [Google Scholar] [CrossRef]
- Lorch, J.M.; Meteyer, C.U.; Behr, M.J.; Boyles, J.G.; Cryan, P.M.; Hicks, A.C.; Ballmann, A.E.; Coleman, J.T.; Redell, D.N.; Reeder, D.M. Experimental infection of bats with Geomyces destructans causes white-nose syndrome. Nature 2011, 480, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, L.; Turner, J.M.; Bollinger, T.K.; Lorch, J.M.; Misra, V.; Cryan, P.M.; Wibbelt, G.; Blehert, D.S.; Willis, C.K. Inoculation of bats with European Geomyces destructans supports the novel pathogen hypothesis for the origin of white-nose syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 6999–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frick, W.F.; Cheng, T.L.; Langwig, K.E.; Hoyt, J.R.; Janicki, A.F.; Parise, K.L.; Foster, J.T.; Kilpatrick, A.M. Pathogen dynamics during invasion and establishment of white-nose syndrome explain mechanisms of host persistence. Ecology 2017, 98, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.J. Mitigating the impacts of agriculture on biodiversity: Bats and their potential role as bioindicators. Mamm. Biol. 2015, 80, 191–204. [Google Scholar] [CrossRef] [Green Version]
- Williams-Guillén, K.; Olimpi, E.; Maas, B.; Taylor, P.J.; Arlettaz, R. Bats in the anthropogenic matrix: Challenges and opportunities for the conservation of Chiroptera and their ecosystem services in agricultural landscapes. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016; pp. 151–186. [Google Scholar]
- O’shea, T.J.; Cryan, P.M.; Hayman, D.T.; Plowright, R.K.; Streicker, D.G. Multiple mortality events in bats: A global review. Mammal. Rev. 2016, 46, 175–190. [Google Scholar] [CrossRef]
- Arnett, E.B.; Baerwald, E.F. Impacts of wind energy development on bats: Implications for conservation. In Bat Evolution, Ecology, and Conservation; Springer: New York, NY, USA, 2013; pp. 435–456. [Google Scholar]
- Hayes, M.A. Bats killed in large numbers at United States wind energy facilities. BioScience 2013, 63, 975–979. [Google Scholar]
- Smallwood, K.S. Comparing bird and bat fatality-rate estimates among North American wind-energy projects. Wildl. Soc. Bull. 2013, 37, 19–33. [Google Scholar] [CrossRef]
- Seto, K.C.; Güneralp, B.; Hutyra, L.R. Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. USA 2012, 109, 16083–16088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, M.L.; Marcot, B.; Mannan, W. Wildlife-Habitat Relationships: Concepts and Applications; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Meyer, C.F.; Struebig, M.J.; Willig, M.R. Responses of tropical bats to habitat fragmentation, logging, and deforestation. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer: Cham, Switzerland, 2016; pp. 63–103. [Google Scholar]
- Brigham, R.M.; Vonhof, M.J.; Barclay, R.M.; Gwilliam, J.C. Roosting behavior and roost-site preferences of forest-dwelling California bats (Myotis californicus). J. Mammal. 1997, 78, 1231–1239. [Google Scholar] [CrossRef]
- Willis, C.K.R.; Brigham, R.M. Physiological and ecological aspects of roost selection by reproductive female hoary bats (Lasiurus cinereus). J. Mammal. 2005, 86, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Kurta, A. Bark roost of a male big brown bat, Eptesicus fuscus. Bat Res. News 1994, 35, 63. [Google Scholar]
- Perry, R.W.; Thill, R.E. Roost selection by male and female northern long-eared bats in a pine-dominated landscape. For. Ecol. Manag. 2007, 247, 220–226. [Google Scholar] [CrossRef]
- Omernik, J.M.; Griffith, G.E. Ecoregions of the conterminous United States: Evolution of a hierarchical spatial framework. Environ. Manag. 2014, 54, 1249–1266. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Goodale, C.L.; Pardo, L.H.; Geiser, L.H.; Lilleskov, E.A.; Robin-Abbott, M.; Driscoll, C. Assessment of Nitrogen Deposition Effects Empirical Critical Loads of Nitrogen for Ecoregions of the United States; US Department of Agriculture, F.S., Northern Research Station: Newtown Square, PA, USA, 2011; Volume 80, pp. 99–116. [Google Scholar]
- Harvey, M.J.; Altenbach, J.S.; Best, T.L. Bats of the United States and Canada; JHU Press: Baltimore, MD, USA, 2011. [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species; Version 2019-3; IUCN: Gland, Switzerland, 2019. [Google Scholar]
- COSEWIC. COSEWIC Assessment and Status Report on the Little Brown Myotis (Myotis lucifugus), Northern Myotis (Myotis septentrionalis), and Tri-colored Bat (Perimyotis subflavus) in Canada; COSEWIC: Ottawa, ON, Canada, 2013. [Google Scholar]
- Hoofer, S. Molecular phylogenetics of the chiropteran family Vespertilionidae. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2003. [Google Scholar]
- Suter, W.; Graf, R.F.; Hess, R. Capercaillie (Tetrao urogallus) and avian biodiversity: Testing the umbrella-species concept. Conserv. Biol. 2002, 16, 778–788. [Google Scholar] [CrossRef]
- Maslo, B.; Leu, K.; Faillace, C.; Weston, M.; Pover, T.; Schlacher, T. Selecting umbrella species for conservation: A test of habitat models and niche overlap for beach-nesting birds. Biol. Conserv. 2016, 203, 233–242. [Google Scholar] [CrossRef]
- Liu, U.; Kenney, S.; Breman, E.; Cossu, T.A. A multicriteria decision making approach to prioritise vascular plants for species-based conservation. Biol. Conserv. 2019, 234, 221–240. [Google Scholar] [CrossRef]
- Betts, B.J. Roosts used by maternity colonies of silver-haired bats in northeastern Oregon. J. Mammal. 1998, 79, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- RC Team. A Language and Environment for Statistical Computing; R Foundation for statistical comuting: Vienna, Austria, 2013. [Google Scholar]
- Suzuki, R.; Shimodaira, H. Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling. R Package, 2.0. 2015. Available online: http://CRAN.R-project.org/package=pvclust (accessed on 10 February 2020).
- McGarigal, K.; Cushman, S.A.; Stafford, S. Multivariate Statistics for Wildlife and Ecology Research; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Badin, H.A. Habitat selection and roosting ranges of northern long-eared bats (Myotis septentrionalis) in an experimental hardwood forest system. Master’s Thesis, Ball State University, Muncie, IN, USA, 2014. [Google Scholar]
- Bergeson, S.M.; Carter, T.C.; Whitby, M.D. Adaptive Roosting Gives Little Brown Bats an Advantage over Endangered Indiana Bats. Am. Midl. Nat. 2015, 174, 321–330. [Google Scholar] [CrossRef]
- Bergeson, S.M.; O’Keefe, J.M.; Haulton, G.S. Managed forests provide roosting opportunities for Indiana bats in south-central Indiana. For. Ecol. Manag. 2018, 427, 305–316. [Google Scholar] [CrossRef]
- Boyles, J.G.; Robbins, L.W. Characteristics of summer and winter roost trees used by evening bats (Nycticeius humeralis) in southwestern Missouri. Am. Midl. Nat. 2006, 155, 210–220. [Google Scholar] [CrossRef]
- Brack, V., Jr.; Whitaker, J.O., Jr.; Pruitt, S.E. Bats of Hoosier National Forest. Proc. Indiana Acad. Sci. 2004, 113, 76–86. [Google Scholar]
- Brack, V. Autumn activity of Myotis sodalis (Indiana bat) in Bland County, Virginia. Northeast. Nat. 2006, 13, 421–434. [Google Scholar] [CrossRef]
- Brandebura, S.C.; Pannkuk, E.L.; Risch, T.S. Indiana Bat (Myotis sodalis) Maternity Colonies in Arkansas. Southeast. Nat. 2011, 10, 529–532. [Google Scholar] [CrossRef]
- Britzke, E.R.; Harvey, M.J.; Loeb, S.C. Indiana bat, Myotis sodalis, maternity roosts in the southern United States. Southeast. Nat. 2003, 2, 235–242. [Google Scholar] [CrossRef]
- Britzke, E.R.; Hicks, A.C.; Von Oettingen, S.L.; Darling, S.R. Description of spring roost trees used by female Indiana bats (Myotis sodalis) in the Lake Champlain Valley of Vermont and New York. Am. Midl. Nat. 2006, 155, 181–187. [Google Scholar] [CrossRef]
- Broders, H.G.; Forbes, G.J. Interspecific and intersexual variation in roost-site selection of northern long-eared and little brown bats in the Greater Fundy National Park Ecosystem. J. Wildl. Manag. 2004, 68, 602–610. [Google Scholar] [CrossRef]
- Callahan, E.V.; Drobney, R.D.; Clawson, R.L. Selection of summer roosting sites by Indiana bats (Myotis sodalis) in Missouri. J. Mammal. 1997, 78, 818–825. [Google Scholar] [CrossRef]
- Carter, T.; Menzel, M.; Chapman, B.; Miller, K. Summer foraging and roosting behavior of an eastern pipistrelle, Pipistrellus subflavus. Bat Res. News 1999, 40, 5–6. [Google Scholar]
- Carter, T.C.; Feldhamer, G.A. Roost tree use by maternity colonies of Indiana bats and northern long-eared bats in southern Illinois. For. Ecol. Manag. 2005, 219, 259–268. [Google Scholar] [CrossRef]
- Carver, B.D.; Ashley, N. Roost Tree Use by Sympatric Rafinesque’s Big-eared Bats (Corynorhinus rafinesquii) and Southeastern Myotis (Myotis austroriparius). Am. Midl. Nat. 2008, 160, 364–373. [Google Scholar] [CrossRef]
- Clement, M.J.; Castleberry, S.B. Southeastern myotis (Myotis austroriparius) roost selection in cypress-gum swamps. Acta Chiropterol. 2013, 15, 133–141. [Google Scholar] [CrossRef]
- Clement, M.J.; Castleberry, S.B. Divergent Roosting Habits of Rafinesque’s Big-eared Bat and Southeastern Myotis During Winter Floods. Am. Midl. Nat. 2013, 170, 158–170. [Google Scholar] [CrossRef]
- Clement, M.J.; Castleberry, S.B. Summer tree roost selection by Rafinesque’s big-eared bat. J. Wildl. Manag. 2013, 77, 414–422. [Google Scholar] [CrossRef]
- Coleman, L.S.; Morris, K.M.; Castleberry, S.B. Characteristics of Lasiurus intermedius (Northern Yellow Bat) Roosts on Sapelo Island, Georgia. Southeast. Nat. 2012, 11, 534–536. [Google Scholar] [CrossRef]
- Duchamp, J.E.; Sparks, D.W.; Whitaker, J.O. Foraging-habitat selection by bats at an urban-rural interface: Comparison between a successful and a less successful species. Can. J. Zool. 2004, 82, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Elmore, L.W.; Miller, D.A.; Vilella, F.J. Selection of diurnal roosts by red bats (Lasiurus borealis) in an intensively managed pine forest in Mississippi. For. Ecol. Manag. 2004, 199, 11–20. [Google Scholar] [CrossRef]
- Fleming, H.L.; Jones, J.C.; Belant, J.L.; Richardson, D.M. Multi-scale Roost Site Selection by Rafinesque’s Big-eared Bat (Corynorhinus rafinesquii) and Southeastern Myotis (Myotis austroriparius) in Mississippi. Am. Midl. Nat. 2013, 169, 43–55. [Google Scholar] [CrossRef]
- Ford, W.M. Summer Roost-Tree Selection by a Male Indiana Bat on the Fernow Experimental Forest; US Dept. of Agriculture, Forest Service, Northeastern Research Station: Newtown Square, PA, USA, 2002; Volume 378. [Google Scholar]
- Ford, W.M.; Owen, S.F.; Edwards, J.W.; Rodrigue, J.L. Robinia pseudoacacia (Black locust) as day-roosts of male Myotis septentrionalis (Northern Bats) on the Fernow Experimental Forest, West Virginia. Northeast. Nat. 2006, 13, 15–24. [Google Scholar] [CrossRef]
- Foster, R.W.; Kurta, A. Roosting ecology of the northern bat (Myotis septentrionalis) and comparisons with the endangered Indiana bat (Myotis sodalis). J. Mammal. 1999, 80, 659–672. [Google Scholar] [CrossRef]
- Gardner, J.E.; Garner, J.D.; Hofmann, J.E. Summer Roost Selection and Roosting Behavior of Myotis sodalis (Indiana bat) in Illinois; INHS Center for Biogeographic Information and IDNR Division of Natural Heritage: Champaign, IL, USA, 1991. [Google Scholar]
- Gardner, J.E.; Garner, J.D.; Hofmann, J.E.; Krejca, J.K.; Robinson, S.E. Distribution and status of Myotis austroriparius (southeastern bat) in Illinois; Illinois Natural History Survey and Illinois Department of Conservation: Champaign, IL, USA, 1992. [Google Scholar]
- Germain, M.J.S.; Kniowski, A.B.; Silvis, A.; Ford, W.M. Who Knew? First Myotis sodalis (Indiana Bat) Maternity Colony in the Coastal Plain of Virginia. Northeast. Nat. 2017, 24, N5–N10. [Google Scholar] [CrossRef]
- Gooding, G.; Langford, J.R. Characteristics of tree roosts of Rafinesque’s big-eared bat and southeastern bat in northeastern Louisiana. Southwest. Nat. 2004, 49, 61–67. [Google Scholar] [CrossRef]
- Gumbert, M.W.; O’Keefe, J.M.; MacGregor, J.R. Roost fidelity in Kentucky; Bat Conservation International: Austin, TX, USA, 2002; pp. 143–152. [Google Scholar]
- Hann, Z.A.; Hosler, M.J.; Moosman, P.R., Jr. Roosting Habits of Two Lasiurus borealis (Eastern Red Bat) in the Blue Ridge Mountains of Virginia. Northeast. Nat. 2017, 24, N15–N18. [Google Scholar] [CrossRef]
- Hein, C.D.; Castleberry, S.B.; Miller, K.V. Sex-specific summer roost-site selection by seminole bats in response to landscape-level forest management. J. Mammal. 2008, 89, 964–972. [Google Scholar] [CrossRef]
- Hein, C.D.; Miller, K.V.; Castleberry, S.B. Evening Bat Summer Roost-Site Selection on a Managed Pine Landscape. J. Wildl. Manag. 2009, 73, 511–517. [Google Scholar] [CrossRef]
- Henderson, L.E.; Broders, H.G. Movements and resource selection of the northern long-eared myotis (Myotis septentrionalis) in a forest-agriculture landscape. J. Mammal. 2008, 89, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Hobson, C.S.; Holland, J.N. Post-hibernation movement and foraging habitat of a male Indiana bat, Myotis sodalis (Chiroptera: Vespertilionidae), in western Virginia. Brimleyana. 1995. Available online: https://hdl.handle.net/1911/21696 (accessed on 10 February 2020).
- Hutchinson, J.T. Bats of Archbold Biological Station and notes on some roost sites. Fla. Field Nat. 2006, 34, 48–51. [Google Scholar]
- Istvanko, D.R.; Risch, T.S.; Rolland, V. Sex-specific foraging habits and roost characteristics of Nycticeius humeralis in north-central Arkansas. J. Mammal. 2016, 97, 1336–1344. [Google Scholar] [CrossRef]
- Jachowski, D.S.; Rota, C.T.; Dobony, C.A.; Ford, W.M.; Edwards, J.W. Seeing the Forest through the Trees: Considering Roost-Site Selection at Multiple Spatial Scales. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Edwards, J.W.; Ford, W.M.; Gates, J.E. Roost tree selection by northern myotis (Myotis septentrionalis) maternity colonies following prescribed fire in a Central Appalachian Mountains hardwood forest. For. Ecol. Manag. 2009, 258, 233–242. [Google Scholar] [CrossRef]
- Johnson, J.B.; Ford, W.M.; Rodrigue, J.L.; Edwards, J.W.; Johnson, C.M. Roost Selection by Male Indiana Myotis Following Forest Fires in Central Appalachian Hardwoods Forests. J. Fish Wildl. Manag. 2010, 1, 111–121. [Google Scholar] [CrossRef]
- Johnson, J.S. Foraging and Roosting Behaviors of Rafinesque’s Big-eared Bat (Corynorhinus rafinesquii) at the Northern Edge of the Species Range. Ph.D. Thesis, University of Kentuck, Lexington, KY, USA, 2012. [Google Scholar]
- Johnson, J.S.; Kropczynski, J.N.; Lacki, M.J.; Langlois, G.D. Social networks of Rafinesque’s big-eared bats (Corynorhinus rafinesquii) in bottomland hardwood forests. J. Mammal. 2012, 93, 1545–1558. [Google Scholar] [CrossRef] [Green Version]
- Kaarakka, H.M. Notes on Capture and Roost Characteristics of Three Female Evening Bats (Nycticeius humeralis) in Southern Wisconsin: An Expanding Species? Am. Midl. Nat. 2018, 180, 168–173. [Google Scholar] [CrossRef]
- Krynak, T.J. Bat habitat use and roost tree selection for northern long-eared myotis (Myotis septentrionalis) in North-Central Ohio. Master’s Thesis, University Heights, Cuyahoga County, OH, USA, 2010. [Google Scholar]
- Kurta, A.; Foster, R.; Hough, E.; Winhold, L. The evening bat (Nycticeius humeralis) on the northern edge of its range—A maternity colony in Michigan. Am. Midl. Nat. 2005, 154, 264–267. [Google Scholar] [CrossRef]
- Kurta, A.; Kath, J.; Smith, E.L.; Foster, R.; Orick, M.W.; Ross, R. A maternity roost of the endangered Indiana bat (Myotis sodalis) in an unshaded, hollow, sycamore tree (Platanus occidentalis). Am. Midl. Nat. 1993, 130, 405–407. [Google Scholar] [CrossRef]
- Kurta, A.; King, D.; Teramino, J.A.; Stribley, J.M.; Williams, K.J. Summer roosts of the endangered Indiana bat (Myotis sodalis) on the northern edge of its range. Am. Midl. Nat. 1993, 129, 132–138. [Google Scholar] [CrossRef]
- Kurta, A.; Williams, K.J.; Mies, R. Ecological, behavioural, and thermal observations of a peripheral population of Indiana bats (Myotis sodalis). Available online: https://www.for.gov.bc.ca/hfd/pubs/Docs/Wp/Wp23/Wp23_11.pdf (accessed on 10 February 2020).
- Lacki, M.J.; Cox, D.R.; Dodd, L.E.; Dickinson, M.B. Response of Northen bats (Myotis septentrionalis) to Prescribed Fires In Eastern Kentucky Forests. J. Mammal. 2009, 90, 1165–1175. [Google Scholar] [CrossRef] [Green Version]
- Leput, D.W. Eastern red bat (Lasiurus borealis) and eastern pipistrelle (Pipistrellus subflavus) maternal roost selection: implications for forest management. Master’s Thesis, Clemson University Clemson, Clemson, SC, USA, 2004. [Google Scholar]
- Lereculeur, A.E. Summer roosting ecology of the northern long-eared bat (Myotis septentrionalis) at Catoosa Wildlife Management Area. Master’s Thesis, Tennessee Technological University, Cookeville, TN, USA, 2013. [Google Scholar]
- Limpert, D.L.; Birch, D.L.; Scott, M.S.; Andre, M.; Gillam, E. Tree selection and landscape analysis of eastern red bat day roosts. J. Wildl. Manag. 2007, 71, 478–486. [Google Scholar] [CrossRef]
- Loeb, S.C. Adaptive response to land-use history and roost selection by Rafinesque’s big-eared bats. J. Mammal. 2017, 98, 560–571. [Google Scholar] [CrossRef]
- Lucas, J.S.; Loeb, S.C.; Jodice, P.G.R. Roost selection by Rafinesque’s big-eared bats (Corynorhinus rafinesquii) in a pristine habitat at three spatial scales. Acta Chiropterol. 2015, 17, 131–141. [Google Scholar] [CrossRef]
- MacGregor, J.R.; Kiser, J.D.; Gumbert, M.W.; Reed, T.O. Autumn roosting habitat of male Indiana bats (Myotis sodalis) in a managed forest setting in Kentucky. In Proceedings of the 12th Central Hardwood Forest Conference, Lexington, KY, USA, 28 February–2 March 1999; USDA Forest Service, Southern Research Station: Asheville, NC, USA, 1999; pp. 169–170. [Google Scholar]
- Mager, K.J.; Nelson, T.A. Roost-site selection by eastern red bats (Lasiurus borealis). Am. Midl. Nat. 2001, 145, 120–126. [Google Scholar] [CrossRef]
- Menzel, M.A.; Carter, T.C.; Chapman, B.R.; Laerm, J. Quantitative comparison of tree roosts used by red bats (Lasiurus borealis) and Seminole bats (L-seminolus). Can. J. Zool. 1998, 76, 630–634. [Google Scholar] [CrossRef] [Green Version]
- Menzel, M.A.; Carter, T.C.; Ford, W.M.; Chapman, B.R. Tree-roost characteristics of subadult and female adult evening bats (Nycticeius humeralis) in the Upper Coastal Plain of South Carolina. Am. Midl. Nat. 2001, 145, 112–119. [Google Scholar] [CrossRef]
- Menzel, M.A.; Carter, T.C.; Ford, W.M.; Chapman, B.R.; Ozier, J. Summer roost tree selection by eastern red, seminole, and evening bats in the upper coast plain of South Carolina. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 2000, 54, 304–313. [Google Scholar]
- Menzel, M.A.; Owen, S.F.; Ford, W.M.; Edwards, J.W.; Wood, P.B.; Chapman, B.R.; Miller, K.V. Roost tree selection by northern long-eared bat (Myotis septentrionalis) maternity colonies in an industrial forest of the central Appalachian mountains. For. Ecol. Manag. 2002, 155, 107–114. [Google Scholar] [CrossRef]
- Miles, A.C.; Castleberry, S.B.; Miller, D.A.; Conner, L.M. Multi-scale roost-site selection by evening bats on pine-dominated landscapes in southwest Georgia. J. Wildl. Manag. 2006, 70, 1191–1199. [Google Scholar] [CrossRef]
- Mormann, B.M.; Robbins, L.W. Winter roosting ecology of eastern red bats in southwest Missouri. J. Wildl. Manag. 2007, 71, 213–217. [Google Scholar] [CrossRef]
- MuÌnzer, O.M. Ecology of the Evening Bat (Nycticeius Humeralis) at the Northern Edge of the Range. Master’s Thesis, Eastern Michigan University, Ypsilanti, MI, USA, 2008; p. 166. [Google Scholar]
- O’Keefe, J.M.; Loeb, S.C.; Lanham, J.D.; Hill, H.S. Macrohabitat factors affect day roost selection by eastern red bats and eastern pipistrelles in the southern Appalachian Mountains, USA. For. Ecol. Manag. 2009, 257, 1757–1763. [Google Scholar] [CrossRef]
- O’Keefe, J.M.; Loeb, S.C.J.F.E.; Management. Indiana bats roost in ephemeral, fire-dependent pine snags in the southern Appalachian Mountains, USA. For. Ecol. Manag. 2017, 391, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Palm, J. Indiana bat (Myotis sodalis) summer roost tree selection and habitat use in the Champlain Valley of Vermont. Doctoral Dissertation, Antioch New England Graduate School, Keene, NH, USA, 2003. [Google Scholar]
- Perry, R.W. Summer roosting by adult male seminole bats in the ouachita mountains, Arkansas. Am. Midl. Nat. 2007, 158, 361–368. [Google Scholar] [CrossRef]
- Perry, R.W.; Brandebura, S.C.; Risch, T.S. Selection of tree roosts by male Indiana bats during the autumn swarm in the Ozark Highlands, USA. Wildl. Soc. Bull. 2016, 40, 78–87. [Google Scholar] [CrossRef]
- Perry, R.W.; Saugey, D.A.; Crump, B.G. Winter Roosting Ecology of Silver-haired Bats in an Arkansas Forest. Southeast. Nat. 2010, 9, 563–572. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E. Roost characteristics of hoary bats in Arkansas. Am. Midl. Nat. 2007, 158, 132–138. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E. Tree roosting by male and female eastern pipistrelles in a forested landscape. J. Mammal. 2007, 88, 974–981. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E. Roost selection by Big Brown Bats in Forests of Arkansas: Importance of Pine Snags and Open Forest Habitats to Males. Southeast. Nat. 2008, 7, 607–618. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E. Diurnal Roosts of Male Evening Bats (Nycticeius humeralis) in Diversely Managed Pine-Hardwood Forests. Am. Midl. Nat. 2008, 160, 374–385. [Google Scholar] [CrossRef]
- Perry, R.W.; Thill, R.E.; Carter, S.A. Sex-specific roost selection by adult red bats in a diverse forested landscape. For. Ecol. Manag. 2007, 253, 48–55. [Google Scholar] [CrossRef]
- Rice, C.L. Roosting Ecology of Corynorphinus Rafinesquii (Rafinesque’s Big-Eared Bat) and Myotis Austroriparius (Southeastern Myotis) in Tree Cavities Found in a Northeastern Louisiana Bottomland Hardwood Forest Streambed. Master’s Thesis, University of Louisiana, Monroe, LA, USA, 2009. [Google Scholar]
- Roby, P.L.; Gumbert, M.W.; Sewell, P.L.; Brewer, S.W. Characteristics of roosts used by Rafinesque’s big-eared bat (Corynorhinus rafinesquii) on camp Mackall, North Carolina. Conserv. Manag. East. Big-Eared Bats—US For. Serv. Gen. Technol. Rep. SRS 2011, 145, 101–110. [Google Scholar]
- Rojas, V.G.; O’Keefe, J.M.; Loeb, S.C. Baseline Capture Rates and Roosting Habits of Myotis septentrionalis (Northern Long-Eared Bat) Prior to White-Nose Syndrome Detection in the Southern Appalachians. Southeast. Nat. 2017, 16, 140–148. [Google Scholar] [CrossRef]
- Sasse, D.B.; Pekins, P.J. Summer roosting ecology of northern long-eared bats (Myotis septentrionalis) in the White Mountain National Forest. In Proceedings of the Bats and Forests Symposium, Victoria, BC, Canada, 19–21 October 1995. [Google Scholar]
- Schaefer, K. Characteristics of Roost Tree Use by the Tri-Colored Bat (Perimyotis subflavus) Post-White-Nose Syndrome in the Four Rivers Watershed. Master’s Thesis 2016. [Google Scholar]
- Schroder, E.S.; Ekanayake, D.B.; Romano, S.P. Indiana bat maternity roost habitat preference within Midwestern United States upland Oak-Hickory (Quercus-Carya) forests. For. Ecol. Manag. 2017, 404, 65–74. [Google Scholar] [CrossRef]
- Silvis, A.; Ford, W.M.; Britzke, E.R.; Beane, N.R.; Johnson, J.B. Forest succession and maternity day roost selection by Myotis septentrionalis in a mesophytic hardwood forest. Int. J. For. Res. 2012, 2012. [Google Scholar] [CrossRef]
- Sparks, D.W.; Ritzi, C.M.; Everson, B.L. Nocturnal behavior and roosting ecology of a juvenile Lasiurus cinereus near Indianapolis, Indiana. Proc. Indiana Acad. Sci. 2005, 114, 70–72. [Google Scholar]
- Stuemke, L.A.; Comer, C.E.; Morrison, M.L.; Conway, W.C.; Maxey, R.W. Roosts of Rafinesque’s Big-Eared Bats and Southeastern Myotis in East Texas. Southeast. Nat. 2014, 13, 159–171. [Google Scholar] [CrossRef]
- Swingen, M.; Baker, R.; Catton, T.; Kirschbaum, K.; Nordquist, G.; Dirks, B.; Moen, R. Preliminary Summary of 2015 Northern Long-eared Bat Research in Minnesota; NRRI Technical Report No. NRRI/TR-2015/44; University of Minnesota Duluth: Duluth, MN, USA, 2015. [Google Scholar]
- Swingen, M.; Baker, R.; Catton, T.; Kirschbaum, K.; Nordquist, G.; Dirks, B.; Moen, R. Summary of 2016 Northern Long-eared Bat Research in Minnesota. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=2ahUKEwi7t_in-PDnAhXUfd4KHR3cBo8QFjAAegQIARAB&url=https%3A%2F%2Ffiles.dnr.state.mn.us%2Feco%2Fnongame%2Fprojects%2Fconsgrant_reports%2F2016%2F2016_swingen.pdf&usg=AOvVaw2jv7phKVVt0KPTmN1xtk2j (accessed on 10 February 2020).
- Thalken, M.M.; Lacki, M.J. Tree roosts of northern long-eared bats following white-nose syndrome. J. Wildl. Manag. 2018, 82, 629–638. [Google Scholar] [CrossRef]
- Timpone, J.C.; Boyles, J.G.; Murray, K.L.; Aubrey, D.P.; Robbins, L.W. Overlap in Roosting Habits of Indiana Bats (Myotis sodalis) and Northern Bats (Myotis septentrionalis). Am. Midl. Nat. 2010, 163, 115–123. [Google Scholar] [CrossRef]
- Timpone, J.C.; Boyles, J.G.; Robbins, L.W. Potential for niche overlap in roosting sites between Nycticeius humeralis (Evening bats) and Eptesicus fuscus (Big brown bats). Northeast. Nat. 2006, 13, 597–602. [Google Scholar] [CrossRef]
- Trousdale, A.W.; Beckett, D.C. Characteristics of tree roosts of Rafinesque’s big-eared bat (Corynorhinus rafinesquii) in southeastern Mississippi. Am. Midl. Nat. 2005, 154, 442–449. [Google Scholar] [CrossRef]
- Veilleux, J.P.; Moosman, P.R.; Reynolds, D.S.; LaGory, K.E.; Walston, L.J. Observations of Summer Roosting and Foraging Behavior of a Hoary Bat (Lasiurus cinereus) in Southern New Hampshire. Northeast. Nat. 2009, 16, 148–152. [Google Scholar] [CrossRef]
- Veilleux, J.P.; Whitaker, J.O.; Veilleux, S.L. Tree-roosting ecology of reproductive female eastern pipistrelles, Pipistrellus subflavus, in Indiana. J. Mammal. 2003, 84, 1068–1075. [Google Scholar] [CrossRef] [Green Version]
- Veilleux, S.L. Tree-roosting Ecology and Natural History of Adult Female Evening Bats, Nycticeius Humeralis, in Indiana. Ph.D. Thesis, Indiana State University, Terre Haute, IN, USA, 2007. [Google Scholar]
- Watrous, K.S.; Donovan, T.M.; Mickey, R.M.; Darling, S.R.; Hicks, A.C.; Von Oettingen, S.L. Predicting minimum habitat characteristics for the Indiana bat in the Champlain Valley. J. Wildl. Manag. 2006, 70, 1228–1237. [Google Scholar] [CrossRef]
- Whitaker, J.O., Jr.; Gummer, S.L. Current status of the evening bat, Nycticeius humeralis, in Indiana. Proc. Indiana Acad. Sci. 2003, 112, 55–60. [Google Scholar]
- Whitaker, J.O., Jr.; Sparks, D.W. Roosts of Indiana bats (Myotis sodalis) near the Indianapolis international airport (1997–2001). Proc. Indiana Acad. Sci. 2008, 117, 193–202. [Google Scholar]
- Lacki, M.J.; Baker, M.D. A prospective power analysis and review of habitat characteristics used in studies of tree-roosting bats. Acta Chiropterol. 2003, 5, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Lacki, M.J.; Cox, D.R.; Dickinson, M. Meta-analysis of summer roosting characteristics of two species of Myotis bats. Am. Midl. Nat. 2009, 318–326. [Google Scholar] [CrossRef]
- Kalcounis-Rüppell, M.C.; Psyllakis, J.M.; Brigham, R.M. Tree roost selection by bats: An empirical synthesis using meta-analysis. Wildl. Soc. Bull. 2005, 33, 1123–1132. [Google Scholar] [CrossRef]
- Naďo, L.; Kaňuch, P. Roost site selection by tree-dwelling bats across biogeographical regions: An updated meta-analysis with meta-regression. Mammal. Rev. 2015, 45, 215–226. [Google Scholar] [CrossRef]
- Humphrey, S.R.; Cope, J.B. Survival rates of the endangered Indiana bat, Myotis sodalis. J. Mammal. 1977, 58, 32–36. [Google Scholar] [CrossRef]
- Humphrey, S.R.; Richter, A.R.; Cope, J.B. Summer habitat and ecology of the endangered Indiana bat, Myotis sodalis. J. Mammal. 1977, 58, 334–346. [Google Scholar] [CrossRef]
- LaVal, R.K.; Clawson, R.L.; LaVal, M.L.; Caire, W. Foraging behavior and nocturnal activity patterns of Missouri bats, with emphasis on the endangered species Myotis grisescens and Myotis sodalis. J. Mammal. 1977, 58, 592–599. [Google Scholar] [CrossRef]
- Cope, J.B.; Humphrey, S.R. Spring and autumn swarming behavior in the Indiana bat, Myotis sodalis. J. Mammal. 1977, 58, 93–95. [Google Scholar] [CrossRef]
- Reynolds, R.J.; Powers, K.E.; Orndorff, W.; Ford, W.M.; Hobson, C.S. Changes in rates of capture and demographics of Myotis septentrionalis (northern long-eared bat) in western Virginia before and after onset of white-nose syndrome. Northeast. Nat. 2016, 23, 195–204. [Google Scholar] [CrossRef]
- Klug, B.J.; Goldsmith, D.A.; Barclay, R.M.R. Roost selection by the solitary, foliage-roosting hoary bat (Lasiurus cinereus) during lactation. Can. J. Zool. 2012, 90, 329–336. [Google Scholar] [CrossRef]
- Betts, B.J. Roosting behaviour of silver-haired bats (Lasionycteris noctivagans) and big brown bats (Eptesicus fuscus) in northeast Oregon. In Bats and Forests Symposium; British Columbia Ministry of Forests: Victoria, BC, Canada, 2000; pp. 55–61. [Google Scholar]
- Jimenez, C. Identifying and Characterizing Roosts of Lasiurus ega and Lasiurus intermedius. Available online: https://asu-ir.tdl.org/handle/2346.1/30538 (accessed on 10 February 2020).
- Spencer, S.G.; Choucair, P.C.; Chapman, B.R. Northward Expansion of the Southern Yellow Bat, Lasiurus ega, in Texas. Southwest. Nat. 1988, 33, 493. [Google Scholar] [CrossRef]
- Waag, A.G. A Novel Approach to Assessing Abundance and Behavior in Summer Populations of Little Brown Myotis in Yellowstone National Park. Master’s Thesis, Ohio University, Athens, OH, USA, 2018. [Google Scholar]
- Fagan, K.E.; Willcox, E.V.; Tran, L.T.; Bernard, R.F.; Stiver, W.H. Roost selection by bats in buildings, Great Smoky Mountains National Park. J. Wildl. Manag. 2018, 82, 424–434. [Google Scholar] [CrossRef]
- Webber, Q.M.; Brigham, R.M.; Park, A.D.; Gillam, E.H.; O’Shea, T.J.; Willis, C.K. Social network characteristics and predicted pathogen transmission in summer colonies of female big brown bats (Eptesicus fuscus). Behav. Ecol. Sociobiol. 2016, 70, 701–712. [Google Scholar] [CrossRef]
- Pfeiffer, M.J. Bats, People, and Buildings: Issues and Opportunities; US Department of Agriculture, Forest Service, Forest Products Laboratory: Madison, WI, USA, 2019; pp. 1–9. [Google Scholar]
- Lacki, M. Restoration of Legacy Trees as Roosting Habitat for Myotis Bats in Eastern North American Forests. Diversity 2018, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Kerth, G.; Weissmann, K.; König, B. Day roost selection in female Bechstein’s bats (Myotis bechsteinii): A field experiment to determine the influence of roost temperature. Oecologia 2001, 126, 1–9. [Google Scholar] [CrossRef]
- Boyles, J.G. Describing roosts used by forest bats: The importance of microclimate. Acta Chiropterol. 2007, 9, 297–303. [Google Scholar] [CrossRef]
- MacPhee, C.; Kershaw, J.A.; Weiskittel, A.R.; Golding, J.; Lavigne, M. Comparison of approaches for estimating individual tree height–diameter relationships in the Acadian forest region. For. Int. J. For. Res. 2017, 91, 132–146. [Google Scholar] [CrossRef]
- Sharma, R.P.; Vacek, Z.; Vacek, S. Modelling tree crown-to-bole diameter ratio for Norway spruce and European beech. Silva Fenn 2017, 51, 1740. [Google Scholar] [CrossRef] [Green Version]
- Otto, M.S.; Becker, N.I.; Encarnacao, J.A. Roost characteristics as indicators for heterothermic behavior of forest-dwelling bats. Ecol. Res. 2016, 31, 385–391. [Google Scholar] [CrossRef]
- Suarez-Rubio, M.; Ille, C.; Bruckner, A.; evolution. Insectivorous bats respond to vegetation complexity in urban green spaces. Ecology 2018, 8, 3240–3253. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.; Kaiser, S.; Böhm, S.; Nieschulze, J.; Kalko, E.K. Moving in three dimensions: Effects of structural complexity on occurrence and activity of insectivorous bats in managed forest stands. J. Appl. Ecol. 2012, 49, 523–531. [Google Scholar] [CrossRef]
- Otto, M.S.; Becker, N.I.; Encarnação, J.A. Stage of pregnancy dictates heterothermy in temperate forest-dwelling bats. J. Therm. Biol. 2015, 47, 75–82. [Google Scholar] [CrossRef]
- Elmore, L.W.; Miller, D.A.; Vilella, F.J. Foraging area size and habitat use by red bats (Lasiurus borealis) in an intensively managed pine landscape in Mississippi. Am. Midl. Nat. 2005, 153, 405–417. [Google Scholar] [CrossRef]
- Thalken, M.M.; Lacki, M.J.; Yang, J. Landscape-scale distribution of tree roosts of the northern long-eared bat in Mammoth Cave National Park, USA. Landsc. Ecol. 2018, 33, 1103–1115. [Google Scholar] [CrossRef] [Green Version]
- Sherman, A. Corynorhinus rafinesquii and Myotis austroriparius artificial roost characteristics in southwestern Mississippi. Master’s Thesis, Jackson State University, Jackson, MS, USA, 2004. [Google Scholar]
- Whitaker, J.O., Jr.; Sparks, D.W.; Brack, V., Jr. Bats of the Indianapolis International Airport Area, 1991–2001. Proc. Indiana Acad. Sci. 2004, 113, 151–161. [Google Scholar]
- Whitaker, J.O.; Gummer, S.L. Population structure and dynamics of big brown bats (Eptesicus fuscus) hibernating in buildings in Indiana. Am. Midl. Nat. 2000, 143, 389–397. [Google Scholar] [CrossRef]
- Riskin, D.K.; Pybus, M.J. The use of exposed diurnal roosts in Alberta by the little brown bat, Myotis lucifugus. Can. J. Zool. 1998, 76, 767–770. [Google Scholar] [CrossRef]
- Thomson, C.E. Myotis sodalis. Mammalian Species 1982, 1–5. [Google Scholar] [CrossRef]
- USFWS. Endangered and threatened wildlife and plants; threatened status for the Northern Long-eared Bat with 4(d) rule. Fed. Regist. 2015, 80, 17974–18033. [Google Scholar]
- Johnson, J.S.; Lacki, M.J. Effects of reproductive condition, roost microclimate, and weather patterns on summer torpor use by a vespertilionid bat. Ecol. Evol. 2014, 4, 157–166. [Google Scholar] [CrossRef]
- Turner, G.G.; Reeder, D.; Coleman, J.T. A Five-year Assessment of Mortality and Geographic Spread of White-Nose Syndrome in North American Bats, with a Look at the Future. Update of White-Nose Syndrome in Bats. Bat Res. News 2011, 52, 13. [Google Scholar]
- Alston, J.M.; Abernethy, I.M.; Keinath, D.A.; Goheen, J.R. Roost selection by male northern long-eared bats (Myotis septentrionalis) in a managed fire-adapted forest. For. Ecol. Manag. 2019, 446, 251–256. [Google Scholar] [CrossRef]
- Silvis, A.; Perry, R.; Ford, W.M. Relationships of Three Species of Bats Impacted by White-Nose Syndrome to Forest Condition and Management; USDA, Ed.; Forest Service: Southern Research Station, Asheville, NC, USA, 2016; Volume 214, pp. 1–48. [Google Scholar]
- Carter, T.C. Indiana bats in the Midwest: The importance of hydric habitats. J. Wildl. Manag. 2006, 70, 1185–1190. [Google Scholar] [CrossRef]





| Latin Binomial | Common Name | IUCN 1 | USFWS 2 | CWS 2 |
|---|---|---|---|---|
| Corynorhinus rafinesquii | Rafinesque’s big-eared bat | LC | NL | NL |
| Eptesicus fuscus | big brown bat | LC | NL | NL |
| Lasionycteris noctivagans | silver haired bat | LC | NL | NL |
| Lasiurus borealis | eastern red bat | LC | NL | NL |
| Lasiurus cinereus | hoary bat | LC | NL | NL |
| Lasiurus intermedius | northern yellow bat | LC | NL | NL |
| Lasiurus seminolus | Seminole bat | LC | NL | NL |
| Myotis austroriparius | southeastern myotis | LC | NL | NL |
| Myotis lucifugus | little brown bat | EN | UR | EN |
| Myotis septentrionalis | northern long-eared bat | NT | TH | EN |
| Myotis sodalis | Indiana bat | NT | EN | NL |
| Nycticeius humeralis | evening bat | LC | NL | NL |
| Perimyotis subflavus | tri-colored bat | VU | UR | EN |
| Bat Species | DBH (cm) | Tree Height (m) | Roost Height (m) | Canopy Closure (%) | Deciduous Roosts % | % Dead Roosts | % Foliage Roosts | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Corynorhinus rafinesquii | 117.8 | (10.9) | 22.4 | (4.7) | 6.3 | (2.5) | 75.2 | (24.8) | 83.4 | 8.4 | 0.0 |
| Eptesicus fuscus | 26.4 | (5.1) | 13.9 | (3.7) | 7.7 | (2.8) | 61 | (4.9) | 22.7 | 86.4 | 0.0 |
| Lasiurus borealis | 29.6 | (5.4) | 19 | (4.4) | 14.4 | (3.8) | 81 | (17.5) | 81.0 | 0.2 | 80.3 |
| Lasiurus cinereus | 35.2 | (5.9) | 22.4 | (4.7) | 21.1 | (4.6) | 80 | (16.3) | 41.2 | 0.0 | 100.0 |
| Lasiurus intermedius | 45.5 | (6.7) | 15.4 | (3.9) | 2.3 | (1.5) | 71.2 | (17.8) | 100.0 | 0.0 | 100.0 |
| Lasiurus seminolus | 40.2 | (6.3) | 26 | (5.1) | 16.3 | (4) | 71.8 | (17.2) | 5.4 | 0.0 | 62.5 |
| Lasionycteris noctivagans | 33.1 | (5.8) | 26.7 | (7.8) | 5.1 | (2.3) | 41.5 | (11.9) | 17.9 | 0.0 | 0.0 |
| Myotis austroriparius | 80.3 | (9) | 23.1 | (4.8) | 1.6 | (1.3) | 87.5 | (11.5) | 79.0 | 11.1 | 0.0 |
| Myotis lucifugus | 40 | (6.3) | 10.7 | (3.3) | 8.4 | (2.9) | 62.4 | (19.4) | 22.1 | 23.5 | 0.0 |
| Myotis septentrionalis | 35.4 | (5.9) | 16.8 | (4.1) | 7.9 | (2.8) | 67.7 | (25.3) | 76.8 | 41.9 | 0.0 |
| Myotis sodalis | 37.7 | (6.1) | 18.3 | (4.3) | 10.1 | (3.2) | 52.5 | (34.9) | 64.9 | 60.9 | 0.0 |
| Nycticeius humeralis | 39.4 | (6.3) | 18 | (4.2) | 11.8 | (3.4) | 67 | (26) | 49.6 | 39.3 | 0.2 |
| Perimyotis subflavus | 27.5 | (5.2) | 20 | (4.5) | 13.7 | (3.7) | 49.1 | (29.7) | 89.5 | 33.1 | 100.0 |
| Discriminant Function | Canopy Closure | Tree Height | Roost Height | DBH | %Deciduous Roosts | %Dead Roosts | %Foliage Roosts |
|---|---|---|---|---|---|---|---|
| DF 1 (69.02%) | −0.00002 | −0.00086 | 0.00336 | −0.0025 | 0.0336 | −0.0382 | 0.6683 |
| DF 2 (21.38%) | 0.00177 | 0.00179 | −0.00713 | 0.00557 | −0.1103 | −0.1491 | 0.17786 |
| Species | EPFU | LANO | MYLU | MYSE | MYSO | NYHU | LABO | LACI | LAIN | LASE | PESU | CORA | MYAU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range (km2) | 2,360,598 | 2,544,272 | 2,237,050 | 1,811,663 | 1,210,854 | 1,958,280 | 2,551,433 | 2,552,372 | 553,416 | 946,051 | 2,432,854 | 1,181,713 | 751,674 |
| Dead Tree Generalists | |||||||||||||
| E. fuscus | 100 | 90.6 | 97.6 | 97 | 99.9 | 100 | - | - | - | - | - | - | - |
| L. noctivagans | 97.7 | 100 | 94.8 | 99.6 | 99.3 | 87.9 | - | - | - | - | - | - | - |
| M. lucifugus | 92.5 | 83.4 | 100 | 98.1 | 99.4 | 81.7 | - | - | - | - | - | - | - |
| M. septentrionalis | 74.5 | 70.9 | 79.4 | 100 | 86.8 | 60 | - | - | - | - | - | - | - |
| M. sodalis | 51.2 | 47.3 | 53.8 | 58 | 100 | 55.9 | - | - | - | - | - | - | - |
| N. humeralis | 83 | 67.7 | 71.5 | 64.8 | 90.5 | 100 | - | - | - | - | - | - | - |
| Foliage Specialists | |||||||||||||
| L. borealis | - | - | - | - | - | - | 100 | 98.1 | 100 | 99.9 | 99.1 | - | - |
| L. cinereus | - | - | - | - | - | - | 98.1 | 100 | 92.1 | 95.4 | 98.1 | - | - |
| L. intermedius | - | - | - | - | - | - | 21.7 | 20 | 100 | 55.9 | 22.1 | - | - |
| L. seminolus | - | - | - | - | - | - | 37 | 35.4 | 95.5 | 100 | 38.2 | - | - |
| P. subflavus | - | - | - | - | - | - | 94.5 | 93.5 | 97.1 | 98.2 | 100 | - | - |
| Southern Wetland Species | |||||||||||||
| C. rafinesquii | - | - | - | - | - | - | - | - | - | - | - | 100 | 91.9 |
| M. austroriparius | - | - | - | - | - | - | - | - | - | - | - | 58.5 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drake, E.C.; Gignoux-Wolfsohn, S.; Maslo, B. Systematic Review of the Roost-Site Characteristics of North American Forest Bats: Implications for Conservation. Diversity 2020, 12, 76. https://doi.org/10.3390/d12020076
Drake EC, Gignoux-Wolfsohn S, Maslo B. Systematic Review of the Roost-Site Characteristics of North American Forest Bats: Implications for Conservation. Diversity. 2020; 12(2):76. https://doi.org/10.3390/d12020076
Chicago/Turabian StyleDrake, Evan C., Sarah Gignoux-Wolfsohn, and Brooke Maslo. 2020. "Systematic Review of the Roost-Site Characteristics of North American Forest Bats: Implications for Conservation" Diversity 12, no. 2: 76. https://doi.org/10.3390/d12020076