- India
- International
Explained: Monoclonal antibodies and Covid-19
Trials in the UK have found a monoclonal antibody cocktail effective in some patients with severe Covid-19. What is this treatment, how does it compare with plasma therapy, and how much promise does it hold?
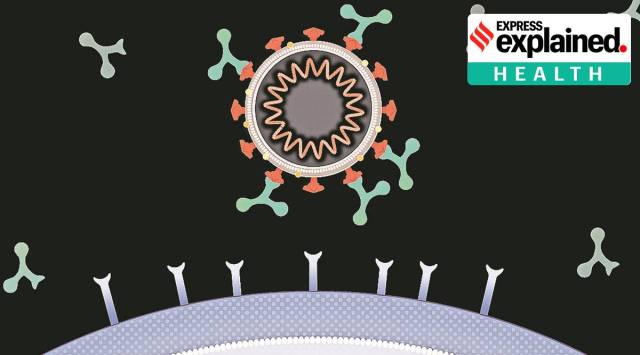 Image of antibodies binding to the surface of the virus. (Source: CoVPN via NIH US)
Image of antibodies binding to the surface of the virus. (Source: CoVPN via NIH US)An experimental monoclonal antibody cocktail, REGEN-COV2, has been found to be a life-saving treatment for some of the most severely affected Covid-19 patients, results of a clinical trial in the UK have shown. How important are the findings for management of Covid-19, including in India?
What are monoclonal antibodies?
To fight a viral infection, our bodies create proteins known as antibodies. Monoclonal antibodies are artificial antibodies that mimic the activity of our immune systems. They are produced through a process that involves extracting specific antibodies from human blood and then cloning them.
These monoclonal antibodies are designed to target a virus or a specific part of one — for instance, REGEN-COV2 is a cocktail of two monoclonal antibodies developed to target the SARS-CoV-2 spike protein. The monoclonal antibodies bind to specific parts of the spike protein, blocking its ability to infect healthy cells.
Besides Covid-19, monoclonal antibodies have been used in the treatment of cancers as well as Ebola and HIV.
How important are they in Covid-19 treatment?
Research during the pandemic has increased optimism in monoclonal antibodies’ ability to help reduce the risk of hospitalisation. Some monoclonal antibodies have shown the ability to retain activity against multiple variants of the virus, suggested Dr Anthony Fauci, Chief Medical Advisor to the US President and Director of the US National Institute of Allergy and Infectious Diseases, during a White House briefing on June 3.

While a crucial and promising part of treatment, monoclonal antibodies also have limitations. So far, these therapies have shown the most success in high-risk groups with mild to moderate Covid-19. They are not approved for use in those hospitalised with severe Covid-19 and those requiring oxygen.
It is very important to provide them to “the right patients at the right time” for the greatest benefits, especially in resource-constrained settings, according to Dr D Behera, a Padma Shri and former HoD at PGIMER Chandigarh’s Department of Pulmonology.
Some emerging variants like the Delta Plus “variant of interest” have also displayed the ability to nullify the use of monoclonal antibodies, according to Dr V K Paul, NITI Aayog’s Member-Health and the Chair of the National Expert Group on Vaccine Administration against Covid-19.
What does the new study show?
The University of Oxford said last week that its RECOVERY trials found that for hospitalised patients with severe Covid-19 “who have not mounted a natural antibody response of their own”, Regeneron’s monoclonal antibody cocktail reduces the risk of death by a fifth compared to those who had received standard care. “Thus, for every 100 such patients treated with the antibody combination, there would be six fewer deaths,” the university said in a release.
The therapy reduced the hospital stay of patients lacking their own natural antibody response by four days. It also reduced their risk of requiring a ventilator. However, “no such benefits were seen in the overall study population”, which includes patients who have been able to mount a natural antibody response.
These findings basically mean that the therapy would be most beneficial for those who have not been able to develop their own antibody response, even if they had developed severe symptoms or been hospitalised.
With 9,785 participants between September 2020 and May 2021, this is the first trial large enough to determine “definitively” whether this treatment reduces death in hospitalised patients with severe Covid-19. This is significant, given that this therapy has so far only been approved for mild to moderate Covid patients.
 Is this therapy available in India?
Is this therapy available in India?
REGEN-COV2 is available in India through a tie-up between Swiss drug giant Roche and Indian company Cipla. The therapy, a combination of monoclonal antibodies casirivimab and imdevimab, had received the Central Drugs Standard Control Organisation’s restricted emergency use permission in May.
In the beginning of June, another antibody cocktail therapy — Eli Lilly’s bamlanivimab and etesevimab —received a similar emergency approval.
Both antibody cocktails are indicated for use in those with mild to moderate Covid-19 who do not require oxygen and who are at a high risk of progressing to severe disease.
GlaxoSmithKline, which on May 26 had announced the US FDA’s Emergency Use Approval for Sotrivimab, is exploring options to make the monoclonal antibody therapy available for India.
In India, Zydus Cadila plans to take an antibody cocktail, ZRC-3308, through trials.
Is it expensive?
Such therapies are expensive because they are difficult to make and take a lot of time. In India, Cipla is supplying 100,000 packs of REGEN-COV2 at a maximum retail price of approximately Rs 1.20 lakh per pack. With one pack offering treatment for two patients, the price of a dose for one patient is Rs 59,750, inclusive of all taxes.
Eli Lilly is engaged in “active dialogue” with the Indian government to “donate” its antibody cocktail for Covid-19 patients.
Monoclonal antibodies have to be made in tissue culture, said Dr Arturo Casadevall, chair of the Department of Molecular Microbiology and Immunology at the Johns Hopkins Bloomberg School of Public Health. “You have to grow the cells. And these cells have to produce the protein which then needs to be purified,” Dr Casadevall said in the School’s “Public Health on Call” podcast on November 2.
How do monoclonal antibodies compare with convalescent plasma therapy?
India last month dropped the use of convalescent plasma as an “off-label” option from its guidance on Covid-19 treatment. Over the last eight months, evidence from trials has shown it has no significant benefits in improving patient outcomes.
Compared with plasma, scientists have expressed more confidence in the promise of monoclonal antibodies. Both antibody-based therapies, they differ in the way they are made.
Convalescent plasma therapy involves providing antibodies from a recovered Covid-19 patient’s plasma. This means that those receiving this therapy would be getting all the antibodies the recovered patient has made.
Monoclonal antibodies are when you take a specific antibody and mass-manufacture it in a factory. For antibody cocktails, you provide a combination of two or more such antibodies.
Monoclonal antibodies are “extremely pure” due to their homogenous nature, Dr Fauci told MedPage Today in August. “The difference between monoclonal antibodies and convalescent plasma is (that) plasma has a lot of other things in it, which could lead to allergic and other reactions,” Fauci said. The data from clinical trials of monoclonal antibodies at that time indicated they are a “very promising form of prevention and treatment,” Dr Fauci had said.
More Explained
EXPRESS OPINION
Apr 26: Latest News
- 01
- 02
- 03
- 04
- 05