Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel betacoronavirus that emerged in Wuhan, China, in late December 2019, rapidly spread across the globe causing the COVID-19 pandemic.
Global scientific collaborations helped the rapid development of multiple vaccines against SARS-CoV-2. Many vaccine modalities, including DNA, mRNA, protein subunit, nanoparticle, viral vectored, and inactivated virus, were trialed, and several of them were subsequently granted emergency authorization for use against COVID-19.
Immune escape and other challenges with currently available vaccines
The emergence of several SARS-CoV-2 variants of concern with varying degrees of resistance to neutralization by convalescent sera is giving rise to concerns of vaccine escape. This highlights the need for more intensive vaccine research and development.
Additionally, some vaccines require ultra-low temperatures during storage, making transport and distribution difficult, especially in developing nations. In light of these challenges, meeting the demand for global vaccination doses has been difficult. Dose-sparing methods could therefore be helpful.
A high-density microarray patch that delivers SARS-CoV-2 spike subunit vaccine directly to the skin
Researchers from Australia recently reported a high-density microarray patch used to deliver a SARS-CoV-2 spike subunit vaccine to the skin directly. They used a combination of a SARS-CoV-2 spike protein vaccine candidate and the high-density microarray patch (HD-MAP) vaccine delivery platform. This results in a stable and effective SARS-CoV-2 vaccine candidate. The study is published on the preprint server, bioRxiv.*
The dry-coated vaccine is delivered directly to the immune-rich epidermis and upper dermal layers of the skin using the HD-MAP platform. It simultaneously causes localized cell death and inflammation, which enhances vaccine-induced immunity. This direct delivery system has several benefits, such as reduced cold chain dependence, ease of administration with a possibility of self-administration without any training, no requirement for reconstitution, and enhanced thermostability.
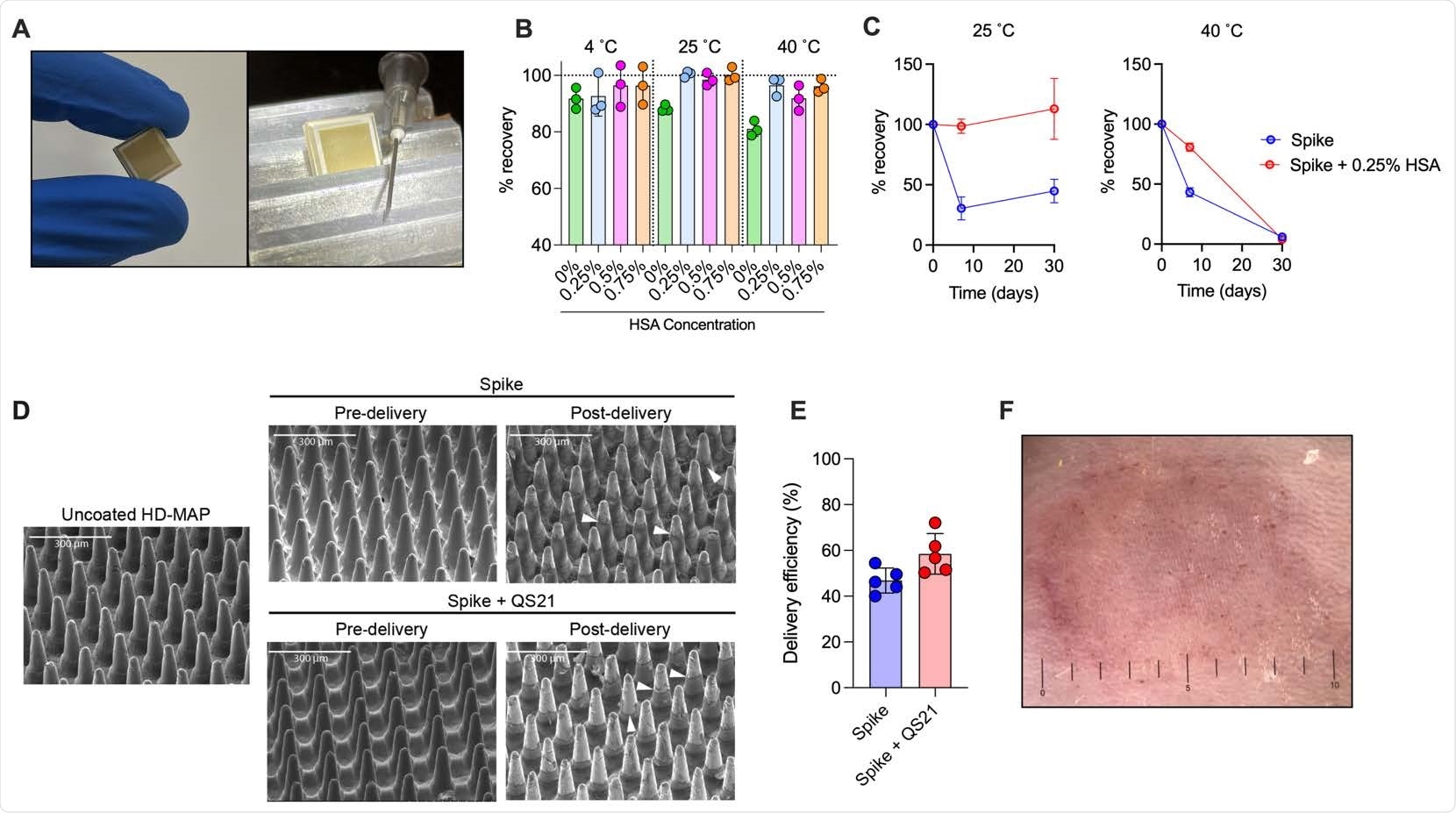
Spike vaccine and the HD-MAP. (A) The HD-MAP relative and 27G needle. Temperature stability of dry-coated spike protein with various concentrations of human serum albumin (HSA) after seven days at the indicated temperatures (B) or with 0.25% HSA for the indicated temperatures and time (C). Data presented as mean with error bars representing SEM. (D)scanning electron microscope images of HD-MAPs either uncoated or coated with spike vaccine formulations, pre- and post-delivery into the skin of mice. White arrowheads indicate levels of vaccine removal post-delivery. (E) Delivery efficiency into the skin of mice of spike and spike + QS21-coated HD-MAPs. (F) HD-MAP delivery site on the flank of a mouse immediately after HD-MAP application and removal.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The thermostable dry-coated spike protein is superior to other vaccine candidates currently available
The dry-coated spike protein was thermostable for up to a week at 40˚C and a month at 25˚C. This, when translated to biophysical stability, represents a significant improvement over other vaccine candidates that have limited stability of 2-24 h at room temperature. This makes it easy to transport and deliver this new vaccine to patients, especially in resource-limited, low-to-middle-income countries.
The delivery of spike protein via HD-MAP elicited higher cellular and antibody immune responses compared to other vaccines. The serum was able to potently neutralize clinically relevant isolates, including the ones from the B.1.1.7 and B.1.351 variants.
“HD-MAP spike vaccines are stable, immunogenic, and protective against virus challenge in mice after a single dose.”
Single-dose of dry patch spike vaccine offers full protection from a fatal virus challenge
A single dose of HD-MAP-delivered spike provided full protection from a fatal virus challenge, which shows that HD-MAP delivery of a COVID-19 vaccine is superior to traditional vaccination using a needle and syringe. Thus, it has the potential to impact the ongoing pandemic.
The spike vaccine delivered using the HD-MAP platform induced robust neutralizing IgG and cellular immune response in mice. This finding is consistent with those of previous studies that utilized the HD-MAP platform and observed significant improvements in immunogenicity.
“Cellular immunity to SARS-CoV-2 is an important component of patient recovery from COVID19 and in providing protection from viral variants.”
The inclusion of QS21 in this vaccine patch broadens the immune response and improves cell-mediated immunity. It also induced high levels of spike-specific T cells and enhanced the scale of RBD- and NTD10-specific antibodies.
Overall, this data demonstrates that HD-MAP vaccination could offer enhanced cellular immune responses and an increase in IgG levels, which can limit the vaccine escape potential of emerging viral variants of concern. The authors concluded that their work is the first ever investigation of SARS-CoV-2 spike vaccination using a microarray patch.
“These findings represent a substantial improvement in many areas of SARS-CoV-2 vaccination and offers a promising alternative to currently available vaccines that warrants further investigation in the context of human SARS-CoV-2 infection.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Complete protection by a single dose skin patch delivered SARS-CoV-2 spike vaccine, Christopher L.D. McMillan, Jovin J.Y. Choo, Adi Idris, Aroon Supramaniam, Naphak Modhiran, Alberto A. Amarilla, Ariel Isaacs, Stacey T.M. Cheung, Benjamin Liang, Helle Bielefeldt-Ohmann, Armira Azuar, Dhruba Acharya, Gabrielle Kelly, Germain J.P. Fernando, Michael J. Landsberg, Alexander A. Khromykh, Daniel Watterson, Paul R. Young, Nigel A.J. McMillan, David A. Muller, bioRxiv, 2021.05.30.446357; doi: https://doi.org/10.1101/2021.05.30.446357, https://www.biorxiv.org/content/10.1101/2021.05.30.446357v1
- Peer reviewed and published scientific report.
McMillan, Christopher L. D., Jovin J. Y. Choo, Adi Idris, Aroon Supramaniam, Naphak Modhiran, Alberto A. Amarilla, Ariel Isaacs, et al. 2021. “Complete Protection by a Single-Dose Skin Patch–Delivered SARS-CoV-2 Spike Vaccine.” Science Advances 7 (44). https://doi.org/10.1126/sciadv.abj8065. https://www.science.org/doi/10.1126/sciadv.abj8065.
Article Revisions
- Apr 8 2023 - The preprint preliminary research paper that this article was based upon was accepted for publication in a peer-reviewed Scientific Journal. This article was edited accordingly to include a link to the final peer-reviewed paper, now shown in the sources section.