
Far fewer people are dying from COVID-19 today than in January, but more than 1,000 Americans die from the disease every day – alone at home or in hospitals, gasping for air, suffering heart attacks or slipping silently away.
Though treatment for the sickest patients has improved since the World Health Organization declared COVID-19 a pandemic a year ago, roughly 20% of patients sick enough to be hospitalized still end up in intensive care – a figure that hasn't changed in the past year, said Kevin Tracey, a neurosurgeon and president and CEO of the Feinstein Institutes for Medical Research, the research arm of Northwell Health, New York’s largest health care provider.
And death rates remain concerningly high in ICUs, he said.
Doctors said the care they give is clearly better than it was a year ago, if only because the disease is better understood and hospitals aren't overflowing with desperately sick patients.
"We've gotten much better at managing patients with COVID-19. Much better than back in March (2020)," said Dr. Daniel Griffin, an infectious disease specialist at ProHEALTH Care, which has 300 health care locations in New York.
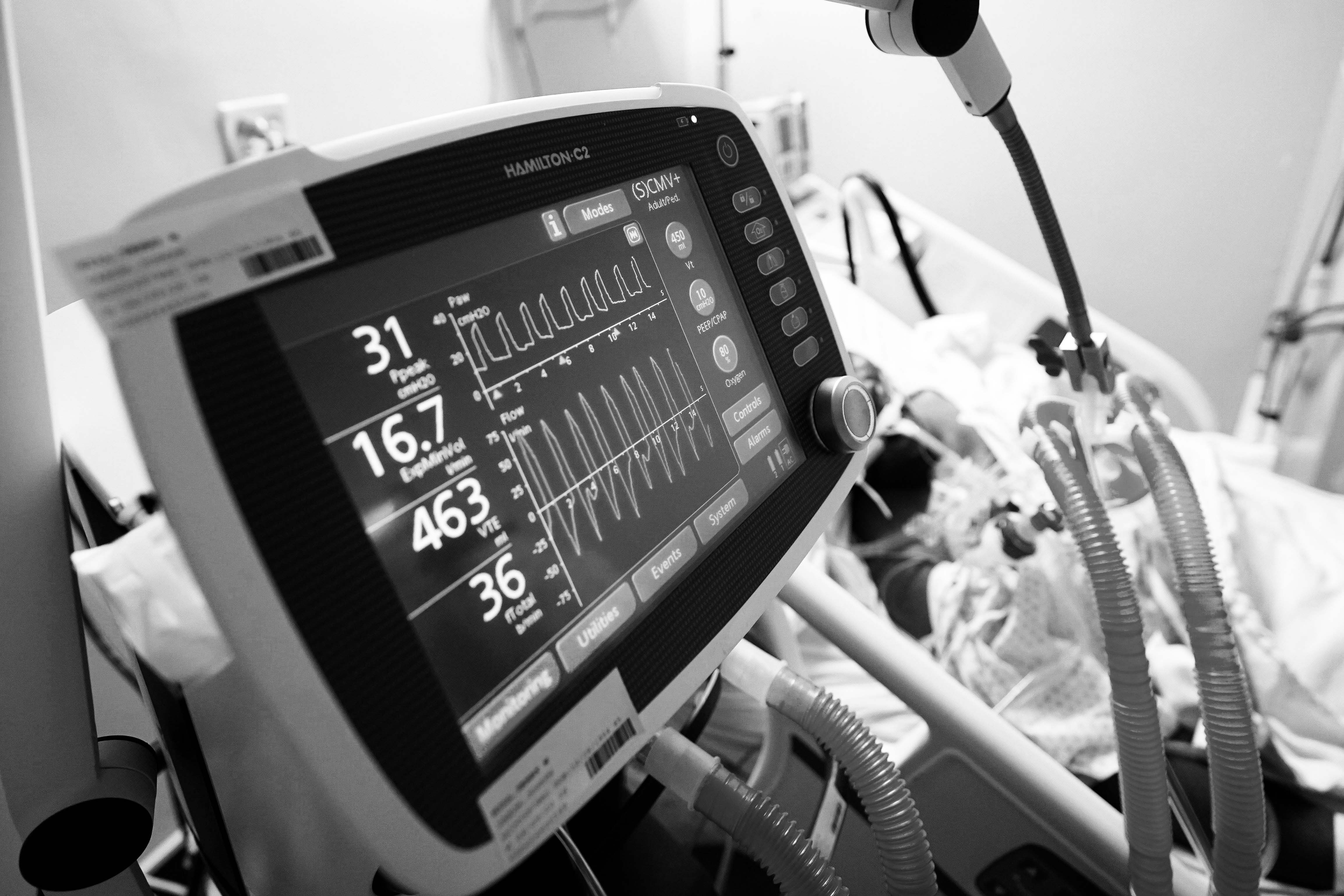
Health workers aren't "throwing the kitchen sink" at patients anymore. They use ventilators more judiciously, finding that delivering oxygen to the throat rather than forcing air into the lungs with a ventilator can be safer and more effective for all but sickest patients. And doctors have one good tool to prevent death – the steroid dexamethasone – which is cheap, effective and easy to use.
The past 12 months have been largely filled with trial and error, rather than systematic learning, doctors said, with little coordinated effort and lots of missed opportunities to turn millions of people's miserable experiences into lessons for others.
"A year later," Tracey said, "we're still flying blind."
Suffering patients and hopeful caregivers have naturally filled the gaps with over-the-counter or readily available options, some of which might be helpful and some hazardous – there's limited research to tell the difference.
The federal government has been running clinical trials in collaboration with private companies, known as the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program to prioritize and speed development of promising treatments.
Those trials have failed to answer key questions, several physician-researchers told USA TODAY.
"Despite all the work that's gone into trying to find a treatment, not only do we not have a lot of options for patients with severe illness, we don't even know what doesn't work," said Dr. Haider Warraich, associate director of the heart failure program at the VA Boston Healthcare System.
The United States lacks a centralized system for running the types of clinical trials that would be needed to prove a drug's effectiveness in patients, particularly when that drug is no longer patented and therefore lacks a champion in the pharmaceutical industry, he and others said.
There was a lack of funding for large, multi-centered trials needed to prove whether drugs do or don't work against severe disease, he and others said. Instead, the system made it easier to give patients therapies with uncertain effectiveness rather than testing those therapies in clinical trials.
"Our response was so fractured, especially in the United States, that we really weren't able to generate any meaningful information," Warraich, said. "I can't but think of the lives that have been lost."

Clinical trials in a pandemic aren't easy
Randomized, placebo-controlled clinical trials are the gold standard for determining the effectiveness of new drugs, but they can be tricky to pull off, especially during a pandemic.
Drs. David Leaf, and Shruti Gupta, kidney specialists at Brigham and Women's Hospital in Boston, focused on collecting information on how some of the sickest COVID-19 patients fared at hospitals across the country.
Last March and April, Leaf and Gupta used their personal connections to get friends and colleagues at 67 other hospital systems nationwide to collect data on COVID-19 patients in intensive care. They created one of the largest independent collaborative networks for studying treatments, called STOP-COVID.
The group has published a dozen studies, including one finding CPR rarely helps COVID-19 patients whose hearts have stopped beating, and another suggesting that ECMO, which temporarily replaces the function of the lungs, can save lives. It found that the anti-inflammatory drug tocilizumab, if given early, appears to save lives – a finding confirmed by large randomized clinical trials.
Without funding, STOP-COVID relied on more than 400 research coordinators, nurses, residents, medical students and physicians across a range of specialties – all volunteers – to review the charts of more than 5,000 patients and hand-enter 800 data points on each. Many times, caregivers would finish a grueling shift in the intensive care unit, then come home and spend unpaid hours entering data.
The grassroots research does not meet the gold standard for clinical research, Leaf conceded. The patients were not randomized to take different therapies, then compared with others who took a placebo.
But the relatively large numbers of very sick patients and robust statistical analysis allowed them to draw reasonable conclusions, Leaf said.
Many of the COVID-19 randomized trials have been too small to definitively determine the effect of treatments on survival.
"Demonstrating an effect on survival is really difficult in these studies unless you have thousands of really sick patients," Leaf said.
It's also been challenging to find dangerously ill patients willing to participate in a trial where they might get a placebo instead of the latest drug to generate a buzz, Tracey said. Many times last spring, he'd get calls in the middle of the night from sobbing family members who'd just learned on the internet about a promising new therapy they wanted to provide to their loved one with COVID-19. The calls continue today.
"How do you argue with that?" Tracey asked. He'd like to stick to science, but "we're all compassionate people and we don't know" what the best approach is.
"We need to chart a route out of this unknown," he said. "We need to start creating knowledge to defeat the ignorance."
It's important to give the right treatment at the right time, which makes it even harder to prove that a treatment works or doesn't, said Dr. David Fajgenbaum, director of the CORONA (COvid19 Registry of Off-label & New Agents) Project, which has been tracking more than 400 drugs given to 270,000 COVID-19 patients.
Running a trial of patients early in the course of their disease, when an antiviral is likely to work best, for instance, is tough with a virus that doesn't cause symptoms for days.
"By the time they come down with symptoms, they've already had COVID for five to 14 days," he said. Researchers "are starting behind the 8-ball."
Public health messages have repeatedly told people to stay home if they've been infected, which works against enrolling them in a trial, he said.
Doctors are allowed to use drugs "off-label" for uses other than what they were approved to treat. Fajgenbaum co-directs the advisory committee of the Cure Drug Repurposing Collaboratory, a federally supported initiative that aims to find other uses for drugs that received U.S. Food and Drug Administration approval.
"Lots of drugs are being tried," Fajgenbaum said. "I'm actually really optimistic about what we've seen so far, and I'm hopeful we build upon that for COVID over these next few months."
Repurposing already approved drugs could help treat thousands of diseases beyond COVID-19, Fajgenbaum said.
"We can save lives with drugs that are already on our shelves," he said. "That's the world we're working towards. I'm actually really hopeful. COVID's been awful in every way, but maybe that could be a silver lining."

Finding treatments that work – or don't
There are four basic categories of potential treatments, according to Fajgenbaum, each of which needs to be given at a different time in the disease course.
•Drugs that boost the immune response early in infection, such as monoclonal antibodies, are given while the body is mounting its response to the virus in the first week after infection. Targeted at high-risk people, these are intended to prevent their disease from getting worse.
•Antiviral drugs, such as remdesivir, target the SARS-CoV-2 virus that causes COVID-19. These are believed to be most effective in the early stages of disease, when they can prevent the virus from taking hold and replicating inside human cells.
Anthony Fauci, head of the National Institute of Allergies and Infectious Diseases, said last week that the government needs to take the same strategy with SARS-CoV-2 antivirals as it did with HIV antiviral drugs, which transformed HIV into a treatable condition.

•Drugs such as the steroid dexamethasone that suppress the immune system are given to the sickest hospitalized patients a week or two after symptoms begin, when their biggest problem is likely to be an immune overreaction to the virus, rather than the virus itself.
•Finally, there are drugs that treat symptoms of COVID-19, such as blood clots, which can theoretically be prevented with the blood thinner heparin, though much of this research is inconclusive.
It's important to use different drugs at different stages of the disease, Fajgenbaum and others said. Tamp down the immune system too early and the virus could wreak havoc; fail to stop an immune overreaction and the patient could die.
"If you modulate the immune response, you can save lives," he said. "It's not about the virus in patients who die, it's about your immune response."
Not understanding that could have dire consequences.
"Giving drugs that don't work – there's a lot of reasons why you shouldn't do that," Fajgenbaum said, "one of which is you could have actually given them something that does work and saved their life."

How can treatments be improved?
The development of effective vaccines against COVID-19 has been a tremendous triumph. "Nothing like this has ever happened before in the history of science," Tracey of Northwell Health said.
He credits investments made over the past 30 years for laying the scientific groundwork that allowed researchers to move so fast on COVID-19 vaccines.
That's not enough to save everyone from the pandemic. Even with vaccines that are 95% effective, 5% of recipients are still vulnerable to symptomatic disease. Out of the 32 million who've received two vaccines, that would be 1.6 million people.
They probably provide some protection against severe disease, but the millions of people who decline vaccines won't have any protection, and some of them will certainly fall ill.
Making effective antivirals against COVID-19 should be a top U.S. priority, Tracey said. Fauci echoed the same sentiment. A good antiviral could prevent exposure to COVID-19 from progressing to full-blown disease and could keep people out of hospitals, intensive care units and morgues.
The country needs a national clinical trials network, ready to spring into action if there's another pandemic, said Dr. R. Scott Wright, director of the human research protection program at the Mayo Clinic in Rochester, Minnesota. It shouldn't be just a government effort, he said, but should include academic medical centers, as well as private hospitals.
"It should be part of the infrastructure moving forward," he said. "It shouldn't be used by groups just to make money, but it ought to be available for emergencies when we have them."
Wright said the failure to effectively treat the opioid epidemic reinforces the need for such a network. "We didn't have a single unifying force" for treating either opioids or COVID-19, he said. "We have been burned twice. It should not happen a third time."
This crisis should be a wake-up call, Tracey said, that the United States needs stockpiles of protective equipment such as masks and gloves, manufacturing capacity and a "doubling down on our investment in research, so we can repopulate the universe of researchers that we amputated in the last 10 years."
"My hope is that this is the Sputnik moment for U.S. science," Tracey said, alluding to the Russian space program that spurred America's race to the moon. "If this doesn't shake us awake as a national security issue, I don't know what will."
Contact Karen Weintraub at kweintraub@usatoday.
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.