Targeting a cell surface vitamin D receptor on tumor-associated macrophages in triple-negative breast cancer
Abstract
Triple-negative breast cancer (TNBC) is an aggressive tumor with limited treatment options and poor prognosis. We applied the in vivo phage display technology to isolate peptides homing to the immunosuppressive cellular microenvironment of TNBC as a strategy for non-malignant target discovery. We identified a cyclic peptide (CSSTRESAC) that specifically binds to a vitamin D receptor, protein disulfide-isomerase A3 (PDIA3) expressed on the cell surface of tumor-associated macrophages (TAM), and targets breast cancer in syngeneic TNBC, non-TNBC xenograft, and transgenic mouse models. Systemic administration of CSSTRESAC to TNBC-bearing mice shifted the cytokine profile toward an antitumor immune response and delayed tumor growth. Moreover, CSSTRESAC enabled ligand-directed theranostic delivery to tumors and a mathematical model confirmed our experimental findings. Finally, in silico analysis showed PDIA3-expressing TAM in TNBC patients. This work uncovers a functional interplay between a cell surface vitamin D receptor in TAM and antitumor immune response that could be therapeutically exploited.
Introduction
Breast cancer is the second most common cancer type worldwide, and triple-negative breast cancer (TNBC) comprises up to ~10–20% of all cases. These heterogeneous tumors are clinically aggressive, usually with larger sizes at initial presentation, of high pathological grade, and likely to have lymph node involvement and early recurrence in visceral sites (Dietze et al., 2015; Newman and Kaljee, 2017; Schettini et al., 2016). TNBC is treated with multimodality therapy including neoadjuvant chemotherapy, surgery and adjuvant radiotherapy, with selected patients receiving additional adjuvant systemic therapy. Despite optimal management, many patients have distant metastases and poor disease outcomes (Biswas et al., 2016; Coughlin, 2019; Dent et al., 2007; Perou et al., 2000). Combination chemotherapy has long been the standard therapeutic option but checkpoint inhibitors and poly ADP-ribose polymerase (PARP) inhibitors have recently been approved in certain settings (Garrido-Castro et al., 2019; Khan et al., 2019; Lyons and Traina, 2019; Marra et al., 2019).
Immunomodulators are among the best available investigational drugs for this tumor subtype, based on the premise that manipulation of the local and/or distant immune responses may ultimately represent a viable treatment approach (Marra et al., 2019). A biological hallmark of TNBC is an immunosuppressive tumor microenvironment that fosters tumor growth and metastatic spread through the suppression of tumor-infiltrating lymphocytes and secretion of immunoinhibitory cytokines, mainly by tumor-associated macrophages (TAM) (DeNardo and Ruffell, 2019; Lim et al., 2018; Wagner et al., 2019). TAM are classically divided into two major populations, M1 and M2, representing the extremes of a broad activation state spectrum; the M1 population is associated with antitumor activity while the M2 population with tumor progression (DeNardo and Ruffell, 2019; Lim et al., 2018; Wagner et al., 2019; Biswas and Mantovani, 2010; Tan et al., 2019). Such biological behavior in breast cancer has made them potentially attractive targets for therapeutic intervention. In fact, TAM-targeting drugs are currently in clinical trials but have not yet been approved for clinical practice.
Results
Combinatorial phage display screening in vivo reveals tumor microenvironment-binding peptides in a mouse model of TNBC
We used a phage display-based approach to identify homing peptides that target TAM in TNBC. The EF43.fgf4 syngeneic mouse mammary gland tumor (Adams et al., 1987; Hajitou et al., 1998) is highly infiltrated by TAM and also serves as an immunocompetent TNBC model since EF43.fgf4 cells do not express the estrogen receptor, progesterone receptor, or Erbb2/Neu (Figure 1—figure supplement 1A). A random phage peptide library was first administered intravenously (iv) in immunocompetent female BALB/c mice with established EF43.fgf4-derived mammary fat pad tumors. Phage particles were recovered from tumors after 24 hr, re-amplified, and subjected to two additional rounds of in vivo selection. After the third round, the pool of tumor-homing phage showed an ~300 fold enrichment relative to normal tissues (Figure 1A). Bioinformatic analysis of peptides targeting the whole tumor revealed four sequences above an experimental threshold (set at 1%): CSSTRESAC, CRYSAARSC, CRGFVVGRC, and CQRALMIAC (Figure 1—figure supplement 1B). Notably, the dominant peptide CSSTRESAC was more strongly enriched (16-fold) than each of the other three peptides (Figure 1B). The four selected peptides were next individually evaluated based on absence of binding to EF43.fgf4 cells in vitro (Figure 1B). With a standard cell binding assay (Giordano et al., 2001), we found that the peptides CRGFVVGRC, CQRALMIAC, and CRYSAARSC bound to EF43.fgf4 cells, whereas the peptide CSSTRESAC did not (Figure 1B), indicating that CSSTRESAC might indeed recognize non-malignant stromal cells within the tumor microenvironment. The peptides CRGFVVGRC, CQRALMIAC, and CRYSAARSC were not studied further.
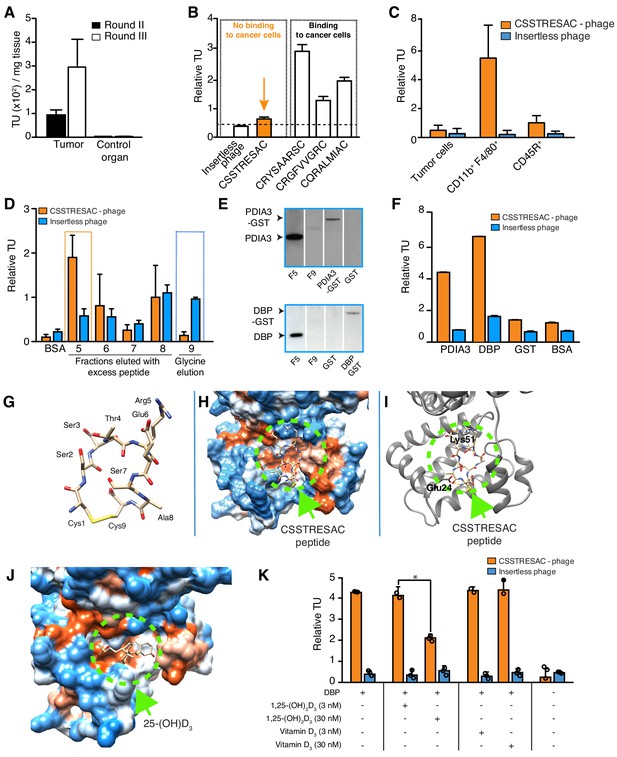
Combinatorial targeting of the tumor cellular microenvironment in a mouse model of TNBC.
(A) A random phage display peptide library displaying CX7C inserts (C, cysteine; X any seven residues) was used in vivo to select peptides homing to the microenvironment of EF43.fgf4-derived mammary tumors. Three sequential rounds of selection resulted in a pool of targeted phage particles with a 300-fold enrichment in the tumor, compared to a control organ (muscle). (B) Binding of individual phage clones to EF43.fgf4 cells was quantified by the counting of transducing units (TU) after host bacterial infection. (C) Binding of CSSTRESAC-phage to EF43.fgf4 tumor cells and non-malignant stromal cell subpopulations isolated from mCherry-expressing EF43.fgf4-derived mammary tumors. (D) Relative binding of the CSSTRESAC-phage or insertless control phage to fractions eluted from a CSSTRESAC-conjugated affinity purification column. BSA was used as negative control protein. (E) Immunoblottings developed with either anti-PDIA3 (top panel) or anti-DBP (lower panel) antibodies show the presence of both affinity-purified proteins in the experimental fraction F#5 but not in the negative control fraction F#9. Human recombinant PDIA3-GST and DBP-GST were used as control for antibody specificity. (F) Phage-binding assay confirms preferential binding of targeted CSSTRESAC-phage to the recombinant human DBP. GST and BSA were used as negative controls. (G) Predicted structure of CSSTRESAC peptide, including a 2.0 Å-disulfide bridge between Cys1 and Cys9, as visualized with UCSF Chimera. (H) Predicted binding conformation and orientation of CSSTRESAC relative to the crystal structure of DBP in a hydrophobicity surface view (PDB ID: 1KW2_A). Orange and blue represent hydrophobic and hydrophilic patches, respectively. (I) Key predicted non-hydrophobic interactions between CSSTRESAC and DBP (PDB ID: 1KW2_A), including a 2.9 Å-salt bridge between Cys1 and Glu24, a 2.9 Å-salt bridge between Glu6 and Lys51, and a 2.9 Å-hydrogen bond between Ala8 and Glu24. CSSTRESAC also blocks access to Tyr48 and Ser92 (Tyr32 and Ser76 in PDB ID: 1J78), which correspond to predicted key residues of DBP interaction with 1,25-(OH)2D3. (J) Crystal structure of 25-(OH)D3 bound to DBP in a hydrophobicity surface view (PDB ID: 1J78). Orange and blue represent hydrophobic and hydrophilic patches, respectively. (K) Binding of CSSTRESAC-phage to DBP is inhibited by the active form of vitamin D [1,25-(OH)2D3], but not by its corresponding vitamin D3 precursor (* represents Student’s t-test, p<0.05).
To identify the non-malignant cellular component(s) targeted by CSSTRESAC-phage, we tested binding to subcellular populations freshly isolated from engrafted tumors. mCherry-expressing EF43.fgf4 cells were FACS-sorted from whole tumors. The remaining cells were subsequently FACS-sorted based on expression of CD45 (Leukocyte Common Antigen, LCA) and F4/80, respectively. Similar to human breast cancers known to be highly infiltrated by macrophages (Biswas et al., 2016; Dent et al., 2007; Perou et al., 2000; Garrido-Castro et al., 2019; Khan et al., 2019; Marra et al., 2019; DeNardo and Ruffell, 2019; Lim et al., 2018; Wagner et al., 2019; Biswas and Mantovani, 2010; Tan et al., 2019; Adams et al., 1987; Hajitou et al., 1998; Giordano et al., 2001), the macrophage population (CD11b+F4/80+) constituted a large portion of the non-malignant cellular component of EF43.fgf4-derived mammary tumors, followed by a lesser population of B lymphocytes (CD45R+). T-lymphocytes (CD8+ or CD4+) were not detected (Figure 1—figure supplement 2). Binding assays to each of these cell subpopulations showed that CSSTRESAC-phage particles bound specifically to CD11b+F4/80+ macrophage; binding to tumor-isolated EF43.fgf4 cells and CD45R+ cells were at background levels (Figure 1C). Based on these results, we concluded that CSSTRESAC-phage particles target a TAM cell surface receptor.
Although we showed that the CSSTRESAC-phage targeted TAM in a syngeneic TNBC model, we considered that it might be able to target the tumor microenvironment in other experimental models of non-TNBC breast cancer also known to be infiltrated by TAM. First, we tested CSSTRESAC-phage homing in the mouse mammary tumor virus-polyoma middle T-antigen (MMTV-PyMT) transgenic model of breast cancer (Guy et al., 1992; Maglione et al., 2001). Binding of the CSSTRESAC-phage to MMTV-PyMT tumors was higher compared to a control organ (~3-fold) or to a negative control phage (~2.5-fold) (Figure 1—figure supplement 3A). To determine whether the CSSTRESAC-phage may also target human tumors, we next used MDA-MB-231-bearing mice, a standard non-TNBC breast cancer xenograft model. We tested whether liposomes decorated with either CSSTRESAC or control peptide could target these tumors by using Magnetic Resonance Imaging (MRI) and fluorescence, and found that CSSTRESAC targets human breast cancers in vivo independently of the phage context. (Figure 1—figure supplement 3B–F). Together, these experiments demonstrate that CSSTRESAC targets a range of different breast tumors (in xenograft, genetic, and syngeneic mouse models) independently of their ligand display context, tumor cell species, or host immunocompetency status. These results also further indicate that the CSSTRESAC peptide may be of value in different types of non-TNBC, and perhaps also other solid tumors containing TAM. Liposome uptake was low in all organs except the liver, a well-known biological phenomenon due to the relatively large size and cationic charge of liposomes (both, targeted or control). None of the liposome preparations caused liver toxicity as confirmed by levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measured in serum of treated mice (Figure 1—figure supplement 4).
Next, we used peptide affinity chromatography (Staquicini et al., 2009) to identify the cell surface receptor(s) in TAM targeted by the CSSTRESAC peptide. Interacting proteins were eluted through an excessive amount of soluble CSSTRESAC peptide and subsequently control acidic glycine buffer. Binding assays were used to identify eluted fractions containing the highest concentrations of receptor(s) (Figure 1D). Proteins present in fraction (F)#5 (positive experimental fraction) and F#9 (negative control fraction) were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and differential protein bands were subjected to in tandem mass spectrometry fragmentation (LS-MS/MS) for protein identification (Supplementary file 1). Notably, immunoblotting of eluted fractions revealed the presence of two vitamin D-binding receptor candidates: protein disulfide-isomerase A3 (PDIA3; also known as glucose-regulated protein-58 kDa, GRP58; endoplasmic reticulum protein of 57 kDa, ERp57; and membrane-associated rapid response to steroid-binding, 1,25D3-MARRS) (Khanal and Nemere, 2007; Figure 1E, top panel) and vitamin D-binding protein (DBP) (Figure 1E, bottom panel). In vitro binding assays to recombinant PDIA3 and DBP confirmed preferential binding of CSSTRESAC-phage relative to the negative control insertless phage (Figure 1F).
CSSTRESAC mimics active vitamin D
PDIA3 and DBP both bind to vitamin D (Christakos et al., 2016), thereby suggesting that CSSTRESAC might be structurally similar to vitamin D. Thus, we applied computational molecular modeling to determine whether the peptide CSSTRESAC would show conformational similarities to vitamin D (Figure 1G–J). The structure of CSSTRESAC was modeled with a de novo peptide structure prediction tool (PEP-FOLD2) (Shen et al., 2014; Figure 1G). Next, Rosetta FlexPepDock (Raveh et al., 2011) was used to identify putative binding site(s) for CSSTRESAC on the surface of DBP. Because the 3D structure of the DBP/1,25-(OH)2D3 complex was not available when this work was performed, we used a 2.3 Å-resolution X-ray crystal structure of the unliganded form of human DBP (PDB ID: 1KW2_A) (Otterbein et al., 2002). To initiate the docking calculation, CSSTRESAC was pre-positioned in the vicinity of the known binding site for 25-(OH)D3, [and likely 1,25-(OH)2D3 based on previous computational modeling], visualized in the 2.1 Å-resolution X-ray crystal structure of a liganded form of human DBP (PDB ID: 1J78) (Verboven et al., 2002). The molecule 25-(OH)D3, also known as calcidiol, binds at the base of a deep, largely hydrophobic pocket on the surface of domain I of DBP (Figure 1J). The computed model of the DBP/CSSTRESAC complex revealed a potential binding site for CSSTRESAC at the opening of the hydrophobic pocket. The computed model suggests that the largely hydrophilic peptide interacts with two superficial residues adjacent to the hydrophobic pocket, including Glu24 and Lys51 (Figure 1H,I; Verboven et al., 2002). The outcome of the Rosetta FlexPepDock calculations suggests that although CSSTRESAC binds at a similar site on the surface of DBP as 1,25-(OH)2D3 and its metabolite calcidiol, it is unlikely to interact more tightly and should be competitively displaced by the natural ligands of the receptor protein (Figure 1I,J). Indeed, experimental binding of CSSTRESAC-phage to immobilized DBP was reduced (Student’s t-test, p<0.05) by increasing amounts of 1,25-(OH)2D3 but not by the non-active precursor vitamin D3 (Figure 1K), a biochemical finding consistent with the computational model.
PDIA3 is a receptor of the CSSTRESAC peptide and a novel molecular marker of TAM
Despite the fact that binding of CSSTRESAC to DBP is strongly suggested by our structural modeling, DBP is a circulating serum protein and thus unlikely to function as an integral cell surface receptor. Therefore, we reasoned that the membrane-bound receptor candidate PDIA3 would likely be the cell surface receptor on TAM responsible for the binding of CSSTRESAC. To determine whether PDIA3 is present on the cell surface of TAM in TNBC, we co-stained CD11b+ TAM isolated from EF43.fgf4 tumors with antibodies against IL-10, IL-12, and PDIA3. Flow cytometry analysis showed robust expression of PDIA3 on the surface of CD11b+IL-10highIL-12low TAM (Figure 2A), identifying PDIA3 as a new cell membrane-associated candidate marker of M2-polarized macrophages. Consistently, EF43.fgf4 cells isolated from tumors did not express PDIA3 (Figure 2B), in agreement with the lack of CSSTRESAC-phage binding to EF43.fgf4 cells. Moreover, immunofluorescence staining of frozen breast tumor sections from tumor-bearing mice receiving CSSTRESAC-phage iv suggested co-localization between PDIA3 and CD68, a well-established cell surface marker of macrophages (Figure 2C,D). Finally, administration of an anti-PDIA3 antibody into EF43.fgf4 tumor-bearing mice confirmed accessibility of PDIA3 through the systemic circulation (Ozawa et al., 2008; Figure 2—figure supplement 1A). Notably, extracellular expression of PDIA3 was largely restricted to resident macrophage in tumors, while control tissues showed minimal cell surface staining. The macrophage marker F4/80 served as an additional positive control (Figure 2—figure supplement 1B).
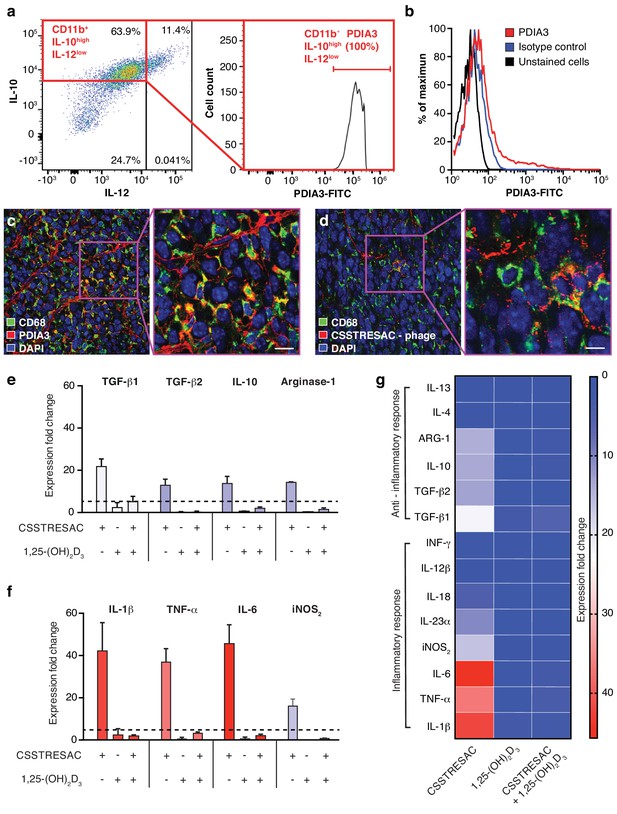
PDIA3 is present on the surface of TAM.
(A) FACS analysis of total TAM isolated from EF43.fgf4-derived mammary tumors shows high levels of PDIA3 expression in a subpopulation of F4/80+CD11b+IL10highIL12low TAM. (B) EF43.fgf4 cells do not express detectable levels of PDIA3 on their surface. (C-D) PDIA3 expression in TAM and co-localization with the pan-macrophage marker CD68 as detected by immunofluorescence of tumor tissue sections from tumor-bearing mice administered iv with anti-PDIA3 antibody (C) or CSSTRESAC-phage (D). (E-G) Purified TAM from EF43.fgf4 mammary tumors were established in culture and treated with either the soluble CSSTRESAC peptide, 1,25-(OH)2D3, or both. Controls included untreated cells, and cells treated with vehicle. Expression of anti-inflammatory (E and G) or pro-inflammatory (F and G) cytokines in CD11b+F4/80+ TAM was assessed by quantitative real-time PCR. Graphics represent expression fold-change relative to control cells.
CSSTRESAC mimics active vitamin D, binds to DBP and mediates activation of PDIA3 on the surface of TAM
To gain insight into the biological mechanisms associated with this newly discovered ligand-receptor system, we next evaluated whether the predicted interactions between CSSTRESAC and PDIA3 on the surface of TAM would have functional consequences. We isolated CD11b+F4/80+ TAM from EF43.fgf4 mammary tumors, established them in culture (>99% purity by FACS), and tested cytokine production as a surrogate for immunoregulatory responses upon treatment (Figure 2E–G). Cytokines were measured by real-time quantitative PCR after RNA extraction from cultured CD11b+F4/80+ TAM exposed to soluble CSSTRESAC. Untreated cultured CD11b+F4/80+ TAM served as negative controls. Treatment of CD11b+F4/80+ TAM with the soluble CSSTRESAC peptide induced a marked (on average ~40 fold) increase in gene expression of the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 (Figure 2F,G). In contrast, there was much lower increases in gene expression of the anti-inflammatory cytokines TGF-β1, TGF-β2, IL-10, and arginase-1 (Figure 2E–G) with IL-4 and IL-13 being undetectable. iNOS2 (~20-fold) and the cytokine IL-23 (~10-fold) were also substantially increased upon exposure to CSSTRESAC. IL-18, IL-12, and INFγ showed modest increases or were detected only at background levels (Figure 2F,G; Figure 2—figure supplement 1C). This cellular response was abrogated when CSSTRESA-treated CD11b+F4/80+ TAM were co-treated with 1,25-(OH)2D3 (Figure 2F,G), verifying that it was specifically caused by the binding of the CSSTRESAC peptide. Thus, binding of CSSTRESAC directly to TAM may alter the local antitumor immune response through changes in cytokine production.
Targeted ablation of PDIA3-expressing TAM affects tumor growth
We next investigated the biological significance and potential therapeutic effects of CSSTRESAC in the EF43.fgf4 tumor model (Figure 3A). Mice bearing size-matched EF43.fgf4 tumors were treated iv with soluble CSSTRESAC peptide, unrelated control peptide, or vehicle. A significant delay in tumor growth of mice treated with CSSTRESAC was observed as soon as one-week post initiation of treatment, compared to tumors of mice receiving an unrelated control peptide or vehicle alone (Figure 3A, t-test, p<0.001). FACS analysis of CD11b+F4/80+ TAM showed a reduction in the number of CD11b+IL10highIL12lowPDIA3-expressing TAM in tumors from mice treated with soluble CSSTRESAC peptide as compared to the negative control groups (Figure 3B and Figure 3—figure supplement 1A). Immunohistochemistry staining of representative tumor sections further demonstrated a reduction of the macrophage population in tumors treated with soluble CSSTRESAC (Figure 3—figure supplement 1B). Thus, treatment of tumors with the soluble CSSTRESAC peptide inhibited tumor growth and altered the TAM population in tumors, which supports it as a potential antitumor drug lead candidate.
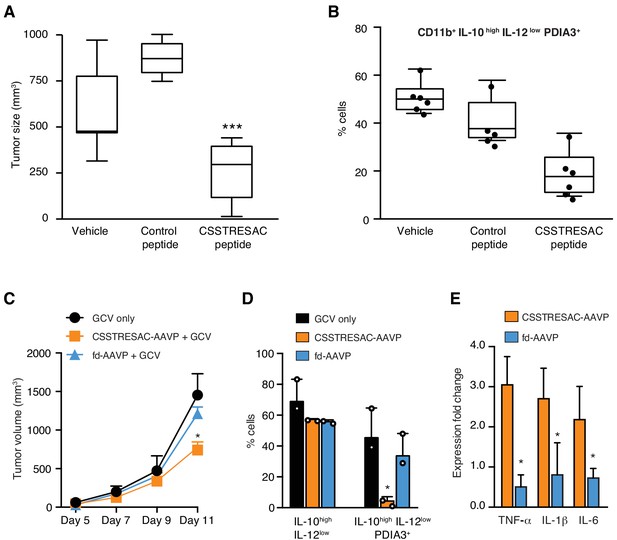
Targeted therapy delays growth of EF43.fgf4-derived mammary tumors.
(A) Therapeutic effect of systemic treatment of EF43.fgf4 tumor-bearing mice with soluble CSSTRESAC peptide (n = 10 each experimental cohort, details in Materials and methods). An unrelated control peptide and vehicle served as negative controls. Tumor sizes were measured by digital caliper 1 week after treatment initiation, and every other day afterwards. *** represents p<0.001. (B) Treatment of tumor-bearing mice with CSSTRESAC reduces the number of PDIA3-expressing TAM (F4/80+CD11b+IL-10highIL-12lowPDIA3+). The TAM population is represented as percentage of total non-malignant cells, as determined by flow cytometry. (C) Gene therapy with CSSTRESAC-AAVP-HSVtk plus GCV delays tumor growth. Mice cohorts with size-matched EF43.fgf4 mammary tumors received a single systemic iv administration of targeted CSSTRESAC-AAVP-HSVtk (5 × 1010 TU) or control fd-AAVP-HSVtk. Mice received daily doses of GCV (80 mg/kg/day) starting at day 7 post AAVP-HSVtk administration until the end of the experiment. * represents p<0.05. (D) Flow cytometry confirms reduction of F4/80+CD11b+IL-10highIL-12lowPDIA3+ TAM in tumors from CSSTRESAC-AAVP-HSVtk-treated mice. (E) Cytokine production by macrophages from tumors of mice treated with CSSTRESAC-AAVP-HSVtk or control groups. * represents p<0.05. Results are reported as expression fold-change relative to control group (set to 1).
As an additional medical application, we also analyzed the use of CSSTRESAC as a theranostic ligand for targeting transgenes directly to tumors in preclinical settings. We engineered adeno-associated/phage (AAVP) (Dobroff et al., 2016; Ferrara et al., 2016; Hajitou et al., 2006; Smith et al., 2016; Staquicini et al., 2011) constructs carrying the Herpes simplex virus thymidine kinase (HSVtk) gene to enable targeted suicide therapy upon combination with the pro-drug ganciclovir (GCV) (Dobroff et al., 2016; Ferrara et al., 2016; Hajitou et al., 2006; Smith et al., 2016; Staquicini et al., 2011; Tjuvajev et al., 1998). CSSTRESAC-AAVP-HSVtk or control AAVP lacking the targeting peptide (fd-AAVP-HSVtk) were delivered to cohorts of size-matched EF43.fgf4 tumor-bearing mice. Animals treated with vehicle were used as controls (n = 10, each cohort). All cohorts received GCV. By the end of the experiment, the sizes of tumors in mice that received CSSTRESAC-AAVP-HSVtk were significantly smaller than that of mice receiving control fd-AAVP-HSVtk or vehicle alone (Figure 3C, t-test, p<0.001). Moreover, macrophage quantification showed a reduction in the number of F480+CD11b+IL10highIL12lowPDIA3-expressing TAM (Figure 3D) accompanied by a shift in the cytokine profile toward an inflammatory response in the tumor microenvironment (Figure 3E and Figure 3—figure supplement 2).
The preclinical efficacies of soluble CSSTRESAC peptide and of CSSTRESAC-AAVP-HSVtk were further investigated in silico. We have conceived a mathematical model of tumor growth and treatment efficiency to predict response in breast cancer patients. This mechanistic model was formulated as a system of ordinary differential equations based on our prior work on modeling cancer response to various forms of drug treatment (Brocato et al., 2018; Brocato et al., 2019; Dogra et al., 2018; Goel et al., 2019; Wang et al., 2016). The model accounts for two primary opposing processes: tumor cell growth and death caused by the CSSTRESAC peptide, while also allowing for competitive antagonism exhibited by 1,25-(OH)2D3 in serum. To model tumor growth delay in gene therapy experiments, an extra death rate term was introduced that characterizes death due to GCV activated through HSVtk (equations are described in Materials and methods). Model predictions corroborated with experimental data from mouse models (Pearson correlation coefficient , p = 0.001) and were used to simulate a clinical trial for treatment of breast cancer patients with soluble CSSTRESAC (Figure 4 and Figure 4—figure supplement 1). To evaluate the importance of a possible competitive binding between 1,25-(OH)2D3 and soluble CSSTRESAC in the serum, the dissociation constant was perturbed by ± 20% of the reference parameter value. As such, an increase in would reflect the competitive binding of the antagonist 1,25-(OH)2D3, where the dissociation of CSSTRESAC from PDIA3 on the cell surface increases and the antitumor effects of CSSTRESAC decreases. Similarly, a reduced would reflect a stronger binding between CCSTRESAC and PDIA3 with the consequent inhibition of tumor growth (Figure 1—figure supplement 1A). We have also considered a hypothetical experiment in patients where a constant rate of i.v. infusion of the soluble CSSTRESAC peptide was compared to the efficacy of a unit i.v. bolus. Our mathematical model predicted that infusion of CCSTRESAC would result in ~400 mm3 greater reduction in tumor volume compared to bolus (Figure 4A and Figure 4—figure supplement 1B). Our proposed working hypothesis shows that the CSSTRESAC-DBP complex specifically binds to PDIA3 and elicits functional changes in PDIA3-expressing TAM within the tumor microenvironment. Such biochemical and cellular alterations may in turn result in an inflammatory local response potentially mediated by IL-6, IL-1β, and TNF-α, and inhibition of tumor growth (Figure 4B).
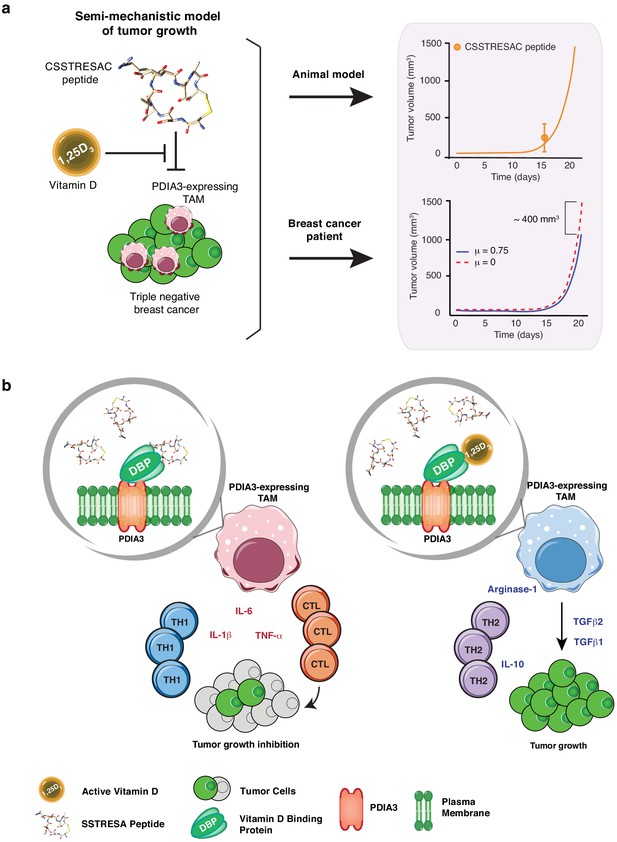
Mechanistic mathematical model of tumor growth inhibition upon treatment with soluble CSSTRESAC and competitive antagonism by 1,25-(OH)2D3.
(A) System interactions captured by a mechanistic mathematical model. Upper panel shows the non-linear regression of the tumor growth model upon treatment of tumor-bearing mice with soluble CSSTRESAC. Error bar represents mean ± standard deviation (S.D.) of the data shown in Figure 3A. Lower panel shows the projected temporal evolution of the tumor volume without infusion (μ = 0) and with infusion (μ = 0.75) in a simulated human clinical trial. (B) A schematic representation of the working hypothesis. The complex CSSTRESAC-DBP binds PDIA3 and eliminates PDIA3-expressing TAM from the tumor microenvironment (through an unknown mechanism), resulting in a pro-inflammatory local response and inhibition of tumor growth. Because 1,25-(OH)2D3 may compete out the effects of CSSTRESAC, binding to PDIA3-expressing TAM in the presence of 1,25-(OH)2D3 may be abrogated, and tumor cells can continue to grow.
Lastly, we searched a publicly available single-cell transcriptome dataset of breast cancer and immune-infiltrating cells containing data from TNBC patients for PDIA3-expressing TAM. Transcripts per million reads (TPM), single-cell (sc)RNA-seq and sample information were obtained from the Gene Expression Omnibus (GEO) repository (accession #GSE75688) (Chung et al., 2017); an initial gene set variation (GSVA) analysis extracted single cells (n = 35) displaying gene expression pathways of infiltrating macrophages. Expression of the PDIA3 gene in these cells was deemed high, medium, or low, and it was clustered/plotted relative to the expression of established markers of immune suppression and M2-polarized macrophages (IL10, TGFB1, CD274, PDCD1LG2, CD68, CD163, ITGAM, CXCL2, and MS4A6A). Markers of angiogenesis and/or disease progression (PLAUR, IL8, VEGFA, and MMP9) were also included (Garrido-Castro et al., 2019; DeNardo and Ruffell, 2019; Lim et al., 2018; Wagner et al., 2019). An unsupervised clustering analysis (Figure 5) showed that high levels of PDIA3 expression in TAM clustered positively with markers of M2-polarized TAM as well as poor prognosis indicators and genes associated with immune suppression. These genomic results support the presence of PDIA3-expressing TAM in human TNBC, and suggest that these preclinical findings may be clinically meaningful.
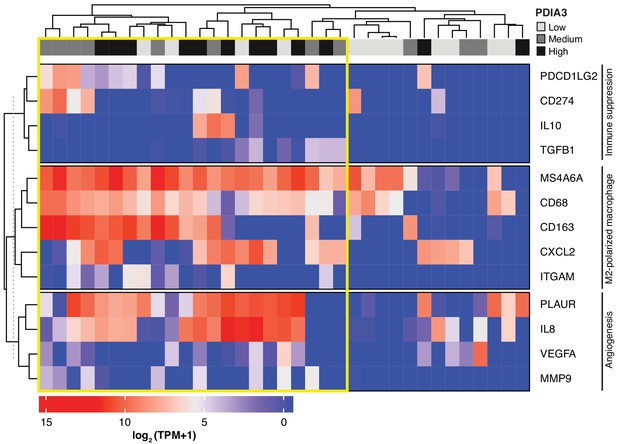
Heat-map of PDIA3 gene expression in pre-defined myeloid cells from human TNBC.
The heat map shows a strong association with the expression of genes characteristic of M2-polarized macrophage, markers of immunosuppression and angiogenesis (i.e. poor prognosis). The yellow box highlights cells with the highest expression of PDIA3.
Discussion
We report that PDIA3 is a functional receptor expressed on the cell surface of the M2-like class of TAM in TNBC. We show that PDIA3, an established vitamin D-interacting protein, has immunoregulatory functions as the TAM cell surface receptor for the peptide CSSTRESAC, with clear effects in preclinical non-TNBC and TNBC mouse models and, at least potentially, in TNBC patients. The effects of soluble CSSTRESAC and CSSTRESAC-AAVP-HSVtk in the local and systemic immune responses in murine models of breast cancer also suggest that combination therapy with immunomodulators may increase the therapeutic response against highly inflammatory tumors. In particular, TNBCs are more likely to respond to immunotherapy due to higher numbers of tumor-infiltrating lymphocytes, higher levels of PD-L1 expression in both tumor cells and immunce cells as well as a higher mutational burden and the consequent rise in tumor-specific neo-antigens. Therefore, immunomodulation of the local and/or systemic responses with immune checkpoint inhibitors could—at least in theory—be amplified by CSSTRESAC-mediated immunoregulatory functions in breast cancer, as well as other TAM-infiltrated cancers, and might perhaps become a medically meaningful translational strategy. In this setting, CSSTRESAC could also be considered as a new non-steroidal vitamin D analogue prototype for drug lead-optimization, with applications that may include other diseases (malignant or non-malignant) with an inflammatory component.
Finally, we introduce a ligand-directed AAVP-HSVtk platform for theranostics based on cell surface targeting of PDIA3 along with a mathematical model that reproduces the experimental dataset and estimates CSSTRESAC treatment outcomes in breast cancer. These observations in vitro, in mouse mammary tumor models, plus an initial in silico analysis of cells from TNBC patients, support an unrecognized regulatory role of PDIA3-expressing TAM in the tumor immune response. Finally, one should note that our mathematical model shows that native competing serum 1,25-(OH)2D3 is unlikely to influence the binding of CSSTRESAC to its target on TAM. Notably, the Human Protein Atlas shows cytoplasmic expression of PDIA3 in human breast cancer cells. Thus, expression of PDIA3 on the surface of cancer cells, and potential effects of the direct binding of CSSTRESAC to breast cancer cells warrants further investigation and, if confirmed, might have translational implications in the setting of TNBC, and other human tumors or even non-malignant disorders with a inflammatory component. Similarly, drug interactions caused by prolonged exposure to CSSTRESAC in the presence of steroids—which are often used in cancer patients—might confound the investigational use of CSSTRESAC-based therapies and should be carefully considered.
Materials and methods
Antibodies and recombinant proteins
Request a detailed protocolAnti-PDIA3, anti-IL-10, anti-IL-12, and anti-F4/80 were purchased from BD Pharmingen and were used in flow cytometry and immunofluorescence. Immunoblottings were performed with antibodies purchased from Sigma (Glutathione S-transferase, PDIA3 and DBP), Abcam (ER), Cell Signaling (PgR), and R and D Systems (HER2). Taqman assays for real-time PCR quantification of cytokines were purchased from Applied Biosystems. Recombinant proteins (DBP and PDIA3), cholecalciferol and calcitriol were all acquired from Abcam. Fluorescence-conjugated secondary antibodies were purchased from Jackson Immunoresearch. Peptides were custom synthesized by PolyPeptide Laboratories to our specifications (>95% purity).
Cells lines and tissue culture
Request a detailed protocolMouse mammary EF43.fgf4 cells (Adams et al., 1987; Hajitou et al., 1998) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 5 ng/ml mouse epithelial growth factor (EGF), 1 μg/ml bovine insulin, and antibiotics. MDA-MB-231 cells (Cailleau et al., 1974) were obtained from the American Type Culture Collection (ATCC) and were grown as a monolayer in RPMI 1640 medium supplemented with 8.25% FBS. Cells were maintained at 37°C and 5% CO2. All cells were routinely tested for the presence of mycoplasm. ATCC garantees the identity of purchased cells.
Animals and experimental tumor models
Request a detailed protocolEight-week-old female nude (nu/nu) mice and immunocompetent BALB/c mice were housed in the animal facilities of the University of Texas M.D. Anderson Cancer Center # 11-99-09935 and Rutgers University New Jersey Medical School (PROTO201800055), all in the USA. Polyoma middle T transgenic (PyMT) mice were maintained at the University Medical Center Hamburg-Eppendorf, Germany. All animal procedures were reviewed and approved by the corresponding Institutional Animal Care and Use Committee (IACUC)-equivalent at each institution.
Both human MDA-MB-231 cells and mouse EF43.fgf4 cells were implanted in the mammary fat pads of nude and immunocompetent BALB/c mice, respectively. Tumor-bearing mice were sorted into experimental size-matched cohorts when established tumors reached ~200 mm3. These procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication #85–23, revised 1996) approved by the local ethics review board.
PyMT [strain FVB/N-TgN (MMTVPyVT)634-Mul] (Michelfelder et al., 2009) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). All procedures involving PyMT mice were conducted in accordance with the German Animal Protection Code and approval was granted by the local ethics review board (Hamburg, Germany). PyMT transgenic mice genotyping was performed through blood samples collected from the retrobulbar venous plexus under anesthesia (2% isoflurane, 98% oxygen), as described (Michelfelder et al., 2009).
Treatment of tumor-bearing mice with a single-dose of CSSTRESAC-AAVP-HSVtk or control fd-AAVP-HSVtk (5 × 1010 TU per mouse) was followed by daily intraperitoneal (ip) administrations of GCV at 80 mg/kg/day. Tumor sizes were measured every-other day with a digital caliper and plotted as tumor volume (mm3).
Phage display methodology
Request a detailed protocolThe Biopanning and Rapid Analysis of Selective Interactive Ligands (BRASIL) methodology (Giordano et al., 2001) was used to test binding of phage to cultured cells. For phage binding to the candidate receptors PDIA3 and DBP, individual microtiter wells of 96-well plates were coated overnight (ON) with 1 μg/ml of recombinant proteins, followed by blocking with BSA and incubation with 109 TU of insertless phage or CSSTRESAC-phage for 1 hr at room temperature (RT). GST and BSA were used as control proteins. Bound phage were recovered by log-phase infection of host bacteria (200 μl E. coli K91Kan). Competitive binding of CSSTRESAC-phage and 1,25-(OH)2D3 to DBP was performed by using the same experimental protocol. Competition was performed in wells pre-incubated with 3 nM or 30 nM of either 1,25-(OH)2D3 or cholecalciferol.
Combinatorial phage display selections in vivo in tumor-bearing mice were performed as described (Dobroff et al., 2016; Ferrara et al., 2016; Hajitou et al., 2006; Smith et al., 2016; Staquicini et al., 2011; Arap et al., 1998; Marchiò et al., 2004; Pasqualini and Ruoslahti, 1996). In brief, animals received 109 TU iv of an unselected phage display random peptide library (displaying the insert CX7C). Tumors and control organs were collected after 24 hr of systemic circulation. For homing of individual phage clones in vivo, tumor-bearing mice were deeply anesthetized with 1–2% isofluorane and received 109 TU of targeted phage or insertless control phage, both administered iv side-by-side. Phage particles were recovered from tissue samples by bacterial infection and processed as described (Dobroff et al., 2016; Ferrara et al., 2016; Hajitou et al., 2006; Smith et al., 2016; Staquicini et al., 2011; Arap et al., 1998; Marchiò et al., 2004; Pasqualini and Ruoslahti, 1996).
Peptide affinity chromatography
Request a detailed protocolReceptor candidates were isolated by using an affinity chromatography CarboxyLink column (ThermoFisher Scientific) conjugated with the synthetic CSSTRESAC peptide. Protein extracts (10 mg/purification) were added to peptide conjugated columns and incubated ON at 4°C under constant gentle agitation. After extensive washes, bound proteins were eluted with an excess of soluble CSSTRESAC peptide followed by elution in low pH glycine buffer. Contaminants including detergents, salts, lipids, phenolics, and nucleic acids were removed through a 2-D clean-up kit from GE Healthcare Life Sciences. Proteins were re-suspended in rehydration buffer (8 M urea, 2% CHAPS, 40 mM DTT, 0.5% IPG buffer, 0.002% bromophenol blue) and 2-D gel electrophoresis was performed by using the ZOOM IPGRunner System (Life Technologies). The final gel was stained with SYPRO Ruby Protein Gel Stain (Life Technologies) and imaged in a 300 nm ultraviolet transilluminator. Unique bands were excised from the SDS gels and digested with trypsin. LC-MS/MS analysis was performed at the Proteomics Core Facility of the University of Texas M.D. Anderson Cancer Center.
To test purified fractions for the presence of candidate receptors, control and experimental fractions were immobilized on individual microtiter wells of 96-well plates ON at 4°C. Wells were blocked with phosphate-buffered saline (PBS) containing 3% BSA for 1 hr at RT and incubated with 109 TU of insertless phage or CSSTRESAC-phage. After extensive washing with PBS, bound phage particles were recovered by infection of host bacteria.
Peptide structure prediction and docking
Request a detailed protocolThe peptide sequence of CSSTRESAC was entered into PEP-FOLD2 (Shen et al., 2014) with a designated disulfide bridge between Cys1 and Cys9 (to ensure the cyclic peptide configuration) and 100- and 200-run simulations were applied. The best-fit model containing a disulfide bridge between Cys1 and Cys9 based on sOPEP energy (i.e. the negative value with greatest absolute value) was selected as the structure for further experimentation. By using the UCSF Chimera (Pettersen et al., 2004), a PDB file with CSSTRESAC positioned adjacent to human DBP (PDB ID: 1KW2_A) (Otterbein et al., 2002) in roughly the same location as 25-(OH)D3 in its complex with human DBP (PDB ID: 1J78) (Verboven et al., 2002) was generated and inputted into Rosetta FlexPepDock (Raveh et al., 2011). The top generated model according to energy scoring, a revised version of Rosetta full-atom and coarse-grained energy functions, with CSSTRESAC bound to the same binding pocket as 25-(OH)D3 was selected for analysis. Interacting residues of CSSTRESAC and DBP were analyzed via UCSF Chimera (Pettersen et al., 2004).
Immunohistochemistry, immunofluorescence, and flow cytometry
Request a detailed protocolFor immunohistochemistry and immunoflorescence, the anti-PDIA3 antibody was administered iv into the tail vein of EF43.fgf4 tumor-bearing BALB/c mice. After 5 min, the mice were killed and perfused through the heart. Tumors and control organs were collected and either quickly-frozen in liquid nitrogen or preservative-fixed, and paraffin-embedded (Dobroff et al., 2016; Ferrara et al., 2016; Hajitou et al., 2006; Smith et al., 2016; Staquicini et al., 2011; Arap et al., 1998; Marchiò et al., 2004; Pasqualini and Ruoslahti, 1996). The presence of the anti-PDIA3 antibody in tissue sections was verified by detection with a secondary antibody conjugated to horseradish peroxidase (HRP) or were stained for the presence of macrophages with an anti-CD68 antibody conjugated to FITC. For flow cytometry, whole EF43.fgf4 tumors were dissected out from tumor-bearing BALB/c mice and single-cell suspensions were prepared by tumor mincing. The single-cell suspension was washed with PBS containing 5% FBS and 0.01% NaN3. Cell suspensions were aliquoted into 12 × 75 mm flow cytometry tubes as 5 × 105 cells per tube and ice-cold incubated for 15 min with an Fc receptor blocking agent, followed by antibodies against PDIA3, F4/80, IL-10, and IL-12. Cells were incubated on ice for 30 min, followed by washes and secondary antibodies.
Quantitative real-time PCR
Request a detailed protocolThree sets of total RNA (RNeasy Mini Kit, Qiagen) were independently isolated from cultured macrophages, or fresh macrophages isolated directly from tumors. DNA synthesis was performed with the GoScript Reverse Transcription System (Promega) by using oligodT for reverse transcription. Gene expression was analyzed with the use of Taqman probes (Applied Biosystems) in a 7500 Fast Real-Time PCR System instrument (Applied Biosystems) and three sets of endogenous control genes: 18S and GAPDH and GUSB1.
Macrophage isolation and tissue culture
Request a detailed protocolTAM were obtained directly from EF43.fgf4 tumors. Tissue digestion was performed in collagenase A in serum-free DMEM (1 mg/mL) for 20 min at 37°C, followed by filtering through 70 μm nylon cell strainers and centrifugation. Macrophages were enriched by magnetic bead separation of CD11b-positive cells (Miltenyi Biotec) and either used for RNA extraction or cultured in 6-well plates containing DMEM (Gibco) supplemented with 20% FBS (Sigma) and 50 ng/ml of M-CSF (R and D Systems). A homogeneous population of adherent macrophages (namely,>99% CD11b+F480+) was obtained after 7 days in culture.
Preparation and characterization of liposomes
Request a detailed protocolCationic lipids DOTAP, DOPE, 1,2-Dioleoyl-sn-Glycero-3-Phosphoethanolamine-N-[4-(p-maleimidophenyl)butyramide] (DOPE-MPB), and DOPE-rhodamine B were purchased from Avanti Polar Lipids. Gadolinium (Gd)-BOA was commercially obtained (Gateway Chemical Technology). Liposomes were prepared by lipid hydration. DOTAP:DOPE:DOPE-MPB (1:0.95:0.05, mol/mol/mol) and were dissolved in chloroform in a round-bottom flask. DOPE-rhodamine B at a concentration of 0.2 mol was included for visualization of liposomes by high-resolution fluorescence microscopy. The total concentration of lipids was determined after extrusion by using the erythrosine method and found to be 10 mg/ml. Gd-BOA (25 mol %) was added to the base formulation in place of different molar fraction of DOTAP. The solvent was removed by evaporation by using nitrogen flow and the lipid film was hydrated by 5% dextrose solution. After hydration, the liposomes were left under an argon blanket at 4°C ON to allow annealing and 24 hr later the suspension of lipids was vortexed for 5 to 10 min, to allow liposome formation, and passed through a 200 nm pore size polycarbonate membrane through an extruder (Avestin Inc). The surface of liposomes was decorated with targeted and control peptides via maleimide chemistry. Fluorescein-labeled control peptide was synthesized by the Synthesis and Sequencing Facility of Johns Hopkins University School of Medicine. A total of 250 μg of either targeted or control peptide was added to the liposomal suspension and left for 24 hr at 4°C to allow covalent coupling. Subsequently, 300 μg of N-ethylmaleimide (NEM; Pierce) were added to the liposomal suspension and kept for 2 hr at RT to block free sulfhydryl groups. Uncoupled peptide and excess of NEM were separated by using Sephadex G-100 size exclusion chromatography.
The hydrodynamic diameters and ζ-potential of liposomes without peptides, liposomes with targeted peptide, or control peptide were measured in 10 mM NaCl at 25°C in two independent experiments in a Malvern Zetasizer (Malvern Inc). Each measurement was repeated at least three times. The total concentration of Gd-BOA incorporated within the lipid bi-layer was determined with inductively coupled plasma mass spectrometry (ICP-MS from Perkin Elmer). The hydrodynamic diameter of liposomes with targeted or control peptides was in the range 150–200 nm with polydispersity index less than 0.2 nm. The presence of either targeted or control peptides on the surface of liposomes did not affect size distribution, and suspensions of liposomes were stable for several months at 4°C. Additional characterization of these liposomes revealed a surface charge of 27.5 ± 2.6 mV for uncoupled liposomes, 26.2 ± 0.9 mV for targeted liposomes, and 24.3 ± 1.2 mV for control liposomes.
Magnetic resonance imaging and optical imaging
Request a detailed protocolCohorts of female nu/nu mice (n = 21) were inoculated in the mammary fat pad with 2 × 106 MDA-MB-231 cells suspended in 50 μl of Hanks balanced salt solution (ThermoFisher Scientific). Prior to tumor implantation, T1 relaxation times of the liposomal solutions were measured on a 4.7 T Bruker Biospec spectrometer horizontal bore magnet (Bruker BioSpin GmBH) with an inversion recovery sequence (repetition time [TR] 2000 milliseconds, number of averages [NA] 1 and 10 relaxation delays of 5, 10, 15, 20, 40, 60, 80, 100, 400, 800 ms). MRI studies were performed when tumor sizes reached ~300–350 mm3. Multi-slice T1-weighted images were acquired with a multislice-spin echo (MSME) sequence (echo time [TE] 11.4 ms, TR 500 milliseconds, NA 2, field of view [FOV] 1.6 cm, matrix size 128 × 128, slice thickness 1 mm, from 6 to 8 slices). Quantitative T1 multi-slice maps with relaxation delays of 100, 500, 1000, and 7000 ms were obtained with TE 0.98 milliseconds, TR 500 ms, NA 8, FOV 1.6 cm, matrix size 128 × 128, slice thickness 1 mm with a modified SNAPSHOT FLASH sequence. The MRI scans were acquired before and at 3, 6, 24, 48, and 72 hr following iv administration of targeted or control liposomes. Images were processed by using customized analyses programs developed in Interactive Data Language (IDL; ITT Visual Information Solutions).
Biodistribution studies of Gd-BOA incorporated within lipid bi-layers of liposomes were performed on an 11.7 T wide-bore MR spectrometer (Bruker BioSpin GmBH) equipped with triple-axis gradients. T1 relaxation times of tumor, liver, kidney, spleen, lungs, intestine, heart, blood, and muscle (n = 3 each) were measured with an inversion recovery sequence (TR = 20 s, NA = 1 and 10 relaxation delays: 5, 10, 15, 20, 40, 60, 80, 100, 400, 800 ms for liver, spleen, and blood, and 40, 80, 100, 200, 400, 600, 1000, 5000, 8000, 10,000 milliseconds for triplicates of tumor, kidney, heart, lungs, and muscle). Biodistribution studies were also performed by using the fluorescent signal from rhodamine-labeled liposomes and FITC-labeled targeted or control peptide. Tumor-bearing mice (n = 3 in each group) received either targeted or control liposomes iv. Mice were killed at each time point and 1-mm-thick slices of tumor, liver, kidney, spleen, lungs, intestine, heart, and muscle were imaged in a Xenogen IVIS 200 optical imaging device (PerkinElmer).
To rule out hepatic toxicity associated to liposomal administration, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) assay kits were purchased from Pointe Scientific Inc Levels of ALT and AST in mice serum were measured 48 hr post iv administration of targeted liposomes, control liposomes, or vehicle-only.
Mathematical model of tumor growth and treatment efficiency
Request a detailed protocolWe developed a mechanistic model of tumor growth formulated as a system of ordinary differential equations based on our prior work on modeling cancer response to various forms of drug treatment (Brocato et al., 2018; Brocato et al., 2019; Dogra et al., 2018; Goel et al., 2019; Wang et al., 2016). The model accounts for two primary opposing processes: tumor cell growth and death caused by the CSSTRESAC peptide, while also allowing for competitive antagonism exhibited by 1,25-(OH)2D3 in serum. To model tumor growth delay in gene therapy experiments, an extra death rate term was introduced that characterizes death due to GCV activated through HSVtk. Specifically, the tumor proliferation rate () is characterized through a logistic equation, the death rate () due to the peptide is modeled as a Michaelis-Menten kinetics process, and the death rate () due to GCV is modeled as a linear function of the concentration of GCV in plasma. Therefore, we obtain the following generic tumor growth model (equations 1 - 4), developed to capture changes in tumor volume () over time:
where is the initial volume of the tumor, is the tumor growth rate constant, is the carrying capacity of the host, is the plasma concentration of CSSTRESAC-AVVP-HSVtk, is the plasma concentration of GCV, is the asymptotic death rate due to the peptide (indicative of potency), is the CSSTRESAC-DBP-PDIA3 complex dissociation constant—which is implicitly a function of the concentration of 1,25-(OH)2D3—and is the proportionality constant between tumor volume and GCV concentration in plasma .
To estimate and for use in the tumor growth model, a one compartment pharmacokinetic (PK) model was employed. Given that the peptide was administered iv, we assumed a first-order renal clearance of the peptide, characterized by an excretion rate constant , such that:
where is the initial plasma concentration of soluble CSSTRESAC.
Further, given that GCV was administered ip, in addition to first-order renal excretion (rate constant ), a first-order absorption of GCV from the peritoneal cavity (rate constant ) into the bloodstream was also incorporated to model its plasma concentration . Hence, we obtain:
where is the concentration of GCV in the peritoneal cavity and is the initial concentration of GCV in the peritoneal cavity. A summary of the system of ordinary differential equations consistent with the dose regimen of the gene therapy experiment is shown below:
In order to perform model parameterization, we began by sequentially fitting the model to the gene therapy data (Figure 3C and Figure 4—figure supplement 1) to estimate the unknown model parameters, which were later used to evaluate the pharmacodynamics of the soluble peptide given in treatment experiments (Figure 3A and Figure 4—figure supplement 1). To estimate the tumor growth rate constant , we solved Equation 1 for in the range of 15– 21 days ignoring the terms and , and used the data corresponding to GCV only (control) to drive a least squares optimization routine. Subsequently, we extrapolated tumor volume to time to obtain , and then used this initial condition to solve Equation 1 for in the range of 0 to 21 days. At this step, the term was retained but was again set to zero, and the data corresponding to fd-AAVP-HSVtk + GCV group was then fit to estimate . Finally, the whole system including the term was solved for 0–21 days and the data corresponding to CSSTRESAC-AAVP-HSVtk + GCV group was used to estimate the parameters , , and . Further, the system of equations 6 and 7 was fit to the literature-derived plasma concentration kinetics of GCV after ip administration in mice to extract the unknown parameters and for use in the tumor growth model. The computed parameters are shown in Supplementary file 2.
In the above calculations, we assumed a mouse weight of 20 g, and a volume of 10 ml per kg for the peritoneal cavity. The administered ip dose of GCV was 80 mg/kg/day, that is, mg/mm3 and the iv administration of the peptide was mM. For all experiments, we used a carrying capacity of 104 mm3. To comply with animal testing regulations, the tumor-bearing mice were killed much before the value 104 mm3 was achieved; we however note that the allowable limit of tumor volumes during in vivo studies does not necessarily reflect the carrying capacity of the host. Hence, a literature-based value for was used (Wu et al., 2018). Of note, in the gene transfer experiments, the peptide is displayed on the AAVP particle, hence it has a different PK behavior (defined by ) than the soluble CSSTRESAC peptide. Therefore, the excretion rate constant of the soluble CSSTRESAC peptide was refit while modeling its pharmacodynamics and was denoted as .
In terms of predictions from the model, we show the fit for in vivo experiments performed in experimental mouse models plus a simulated clinical trial for treatment of human breast cancer with soluble CSSTRESAC (Figure 4A and Figure 4—figure supplement 1). The obtained value of , and the corresponding half-life of CSSTRESAC were computed as , which is ~26 min. Therefore, the half-life of the CSSTRESAC conforms to those of other targeting pharmacological data (Pasqualini et al., 2015) and indicates that soluble CSSTRESAC is rapidly cleared through renal excretion. To evaluate the importance of a possible competitive binding between 1,25-(OH)2D3 and soluble CSSTRESAC in the serum, the dissociation constant was perturbed by ± 20% of the reference parameter value. As such, an increase in would reflect the competitive binding of the antagonist 1,25-(OH)2D3, where the dissociation of CSSTRESAC from PDIA3 on the cell surface increases and the antitumor effects of CSSTRESAC decreases. Similarly, a reduced would reflect a stronger binding between CCSTRESAC and PDIA3 with the consequent inhibition of tumor growth (Figure 4A, Figure 4—figure supplement 1).
We also considered a hypothetical experiment in human patients where a constant rate of iv infusion of the soluble CSSTRESAC peptide was compared to the efficacy of a unit i.v. bolus. In this scenario, the kinetics of the soluble CSSTRESAC peptide is dictated by equation 8. The infusion constant µ denotes the asymptotic concentration of the peptide and is the infusion rate. Our mathematical model predicted that infusion of CCSTRESAC (µ = 0.75) would resulted in greater reduction in tumor volume compared to bolus (Figure 4A and Figure 4—figure supplement 1). Thus, this in silico experiment clearly illustrates the use of a mathematical model in predicting potential therapeutic effects and limitations of soluble CSSTRESAC. Similarly, our mathematical model when fit to the experimental data showed satisfactory agreement as indicated by the Pearson correlation coefficient (p = 0.001) (Figure 4—figure supplement 1). The model parameter estimates are listed (Supplementary file 2).
PDIA3 gene expression in human single cells from TNBC patients
Request a detailed protocolIn order to evaluate the expression levels of PDIA3 mRNA in human breast cancer samples, we obtained the clinical and scRNA-seq data from a publicly available single cell database from TNBC patients (BC07 - BC11) as originally reported (Chung et al., 2017). The scRNA-seq datasets were reported as TPM and were assessed through the GEO repository (accession number GSE75688). Gene expression levels of 35 pre-defined myeloid cells were retrieved and distributed into three groups according to PDIA3 expression levels (set as high, medium, or low). A heat-map was generated to show potential associations between PDIA3 and gene pathways characteristic of macrophages.
Statistical analysis
Request a detailed protocolComparisons among the groups were assessed by One-way ANOVA with SigmaStat (SPSS Inc) and GraphPad Prism (GraphPad Software Inc). Statistical significance was set at a p-value of <0.05 unless otherwise specified. Normally distributed data are shown as bar graphs with means ± standard deviation (SD) or standard error of the mean (SEM) as indicated, whereas not normally distributed data are shown in box-and-whiskers plots: the boxes define the 25th and 75th percentiles, a line denotes the median and error bars define the 10th and 90th percentiles.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files. Source data files have been provided in Materials and methods and Supplementary files.
-
NCBI Gene Expression OmnibusID GSE75688. Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer.
References
-
Enhanced in vitro proliferation and in vivo tumorigenic potential of mammary epithelium from BALB/c mice exposed in vivo to gamma-radiation and/or 7,12-dimethylbenz[a]anthraceneCancer Research 47:4425–4431.
-
Breast tumor cell lines from pleural effusionsJNCI: Journal of the National Cancer Institute 53:661–674.https://doi.org/10.1093/jnci/53.3.661
-
Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effectsPhysiological Reviews 96:365–408.https://doi.org/10.1152/physrev.00014.2015
-
Epidemiology of breast Cancer in womenAdvances in Experimental Medicine and Biology 1152:9–29.https://doi.org/10.1007/978-3-030-20301-6_2
-
Macrophages as regulators of tumour immunity and immunotherapyNature Reviews Immunology 19:369–382.https://doi.org/10.1038/s41577-019-0127-6
-
Triple-negative breast Cancer: clinical features and patterns of recurrenceClinical Cancer Research 13:4429–4434.https://doi.org/10.1158/1078-0432.CCR-06-3045
-
Triple-negative breast Cancer in African-American women: disparities versus biologyNature Reviews Cancer 15:248–254.https://doi.org/10.1038/nrc3896
-
Biopanning and rapid analysis of selective interactive ligandsNature Medicine 7:1249–1253.https://doi.org/10.1038/nm1101-1249
-
Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic diseaseMolecular and Cellular Biology 12:954–961.https://doi.org/10.1128/mcb.12.3.954
-
The ERp57/GRp58/1,25D3-MARRS receptor: multiple functional roles in diverse cell systemsCurrent Medicinal Chemistry 14:1087–1093.https://doi.org/10.2174/092986707780362871
-
Inflammatory breast Cancer biology: the tumour microenvironment is keyNature Reviews Cancer 18:485–499.https://doi.org/10.1038/s41568-018-0010-y
-
Emerging novel therapeutics in Triple-Negative breast CancerAdvances in Experimental Medicine and Biology 1152:377–399.https://doi.org/10.1007/978-3-030-20301-6_20
-
Transgenic polyoma middle-T mice model premalignant mammary diseaseCancer Research 61:8298–8305.
-
Beyond receptor expression levels: the relevance of target accessibility in ligand-directed pharmacodelivery systemsTrends in Cardiovascular Medicine 18:126–133.https://doi.org/10.1016/j.tcm.2008.03.001
-
UCSF chimera--a visualization system for exploratory research and analysisJournal of Computational Chemistry 25:1605–1612.https://doi.org/10.1002/jcc.20084
-
Improved PEP-FOLD approach for peptide and miniprotein structure predictionJournal of Chemical Theory and Computation 10:4745–4758.https://doi.org/10.1021/ct500592m
-
Systemic combinatorial peptide selection yields a non-canonical iron-mimicry mechanism for targeting tumors in a mouse model of human glioblastomaJournal of Clinical Investigation 121:161–173.https://doi.org/10.1172/JCI44798
-
Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomographyCancer Research 58:4333–4341.
-
A structural basis for the unique binding features of the human vitamin D-binding proteinNature Structural Biology 9:131–136.https://doi.org/10.1038/nsb754
-
In silico mouse study identifies tumour growth kinetics as biomarkers for the outcome of anti-angiogenic treatmentJournal of the Royal Society Interface 15:20180243.https://doi.org/10.1098/rsif.2018.0243
Article and author information
Author details
Funding
U.S. Department of Defense (W81XWH-09-1-0224)
- Fernanda I Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela I Staquicini
- Wadih Arap
- Renata Pasqualini
Gilson Logenbaugh Foundation
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Wadih Arap
- Renata Pasqualini
Susan G. Komen
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Wadih Arap
- Renata Pasqualini
National Science Foundation (DBI-1832184)
- Christopher Markosian
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
National Institutes of Health (R01GM133198)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
U.S. Department of Energy (DE-SC0019749)
- Stephen Burley
Fundação de Amparo à Pesquisa do Estado de São Paulo (2012/24105-3)
- Mauro Cortez
National Science Foundation (DMS-1930583)
- Christopher Markosian
- Zhihui Wang
- Vittorio Cristini
National Institutes of Health (1U01CA196403)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
National Institutes of Health (1U01CA213759)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
National Institutes of Health (1R01CA226537)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
National Institutes of Health (1R01CA222007)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
National Institutes of Health (U54CA210181)
- Fernanda Staquicini
- Amin Hajitou
- Wouter HP Driessen
- Bettina Proneth
- Marina Cardó-Vila
- Daniela Staquicini
- Zhihui Wang
- Vittorio Cristini
- Stephen Burley
- Wadih Arap
- Renata Pasqualini
Fundação de Amparo à Pesquisa do Estado de São Paulo (2020/13562-0)
- Mauro Cortez
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This work was supported by the US DOD IMPACT grant W81XWH-09-1-0224 and by serial awards from the Gillson-Longenbaugh Foundation and the Susan G Komen Breast Cancer Foundation (to WA and RP). The RCSB Protein Data Bank is supported by grants to SKB from the National Science Foundation (DBI-1832184), the NIH (R01GM133198), and the US DOE (DE-SC0019749). This work has also been supported by the National Science Foundation Grant DMS-1930583 (ZW, VC), NIH Grants 1U01CA196403 (ZW, VC), 1U01CA213759 (ZW, VC), 1R01CA226537 (ZW, VC, WA, RP), 1R01CA222007 (ZW, VC), and U54CA210181 (ZW, VC). MCo was partially funded by FAPESP (2012/24105-3, 2020/13562–0). We thank Dr. Helen Pickersgill (Life Science Editors) for professional editing and Dr. Webster K Cavenee, Dr. Sylvia Christakos, and Dr. E Helene Sage for critical reading of the manuscript. JGG, WA, and RP are founders and equity stockholders of PhageNova Bio, which has licensed reagents disclosed in this manuscript. AH, WHPD, BP, WA and RP are entitled to royalty payments from this licensing agreement. RP is the Chief Scientific Officer and a paid consultant for PhageNova Bio. WA and RP are partially supported by a Sponsored Research Agreement from PhageNova Bio. AH, JGG, WA, and RP are inventors on issued and pending patent applications related to technology disclosed in this manuscript and will be entitled to royalties if licensing or commercialization occurs. WA and RP are also founders and equity holders of MBrace Therapeutics. RP serves as a Board Member of MBrace Therapeutics. These arrangements are managed and monitored in accordance with the established institutional conflict of interest policies of Rutgers, the State University of New Jersey. MCr is a consultant for CytoDyn, Sermonix Pharmaceuticals, G1 Therapeutics, Foundation Medicine, Dompé, ArcherDX and Menarini. MCr also receives honoraria/travel grants from Pfizer, Lilly, Novartis, Sermonix Pharmaceuticals, Foundation Medicine and Menarini. Other authors declare that they have no competing interests.
Ethics
Animal experimentation: Eight-week-old female nude (nu/nu) mice and immunocompetent BALB/c mice were housed in the animal facilities of the University of Texas M.D. Anderson Cancer Center # 11-99-09935 and Rutgers University New Jersey Medical School (PROTO201800055), all in the USA. All animal procedures were reviewed and approved by the corresponding Institutional Animal Care and Use Committee (IACUC)-equivalent at each institution.
Version history
- Received: November 24, 2020
- Accepted: April 23, 2021
- Version of Record published: June 1, 2021 (version 1)
- Version of Record updated: July 12, 2021 (version 2)
Copyright
© 2021, Staquicini et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,078
- views
-
- 321
- downloads
-
- 18
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Medicine
The nuclear receptor, farnesoid X receptor (FXR/NR1H4), is increasingly recognized as a promising drug target for metabolic diseases, including nonalcoholic steatohepatitis (NASH). Protein-coding genes regulated by FXR are well known, but whether FXR also acts through regulation of long non-coding RNAs (lncRNAs), which vastly outnumber protein-coding genes, remains unknown. Utilizing RNA-seq and global run-on sequencing (GRO-seq) analyses in mouse liver, we found that FXR activation affects the expression of many RNA transcripts from chromatin regions bearing enhancer features. Among these we discovered a previously unannotated liver-enriched enhancer-derived lncRNA (eRNA), termed FXR-induced non-coding RNA (Fincor). We show that Fincor is specifically induced by the hammerhead-type FXR agonists, including GW4064 and tropifexor. CRISPR/Cas9-mediated liver-specific knockdown of Fincor in dietary NASH mice reduced the beneficial effects of tropifexor, an FXR agonist currently in clinical trials for NASH and primary biliary cholangitis (PBC), indicating that amelioration of liver fibrosis and inflammation in NASH treatment by tropifexor is mediated in part by Fincor. Overall, our findings highlight that pharmacological activation of FXR by hammerhead-type agonists induces a novel eRNA, Fincor, contributing to the amelioration of NASH in mice. Fincor may represent a new drug target for addressing metabolic disorders, including NASH.
-
- Cell Biology
- Medicine
Chemotherapy is a widely used treatment for a variety of solid and hematological malignancies. Despite its success in improving the survival rate of cancer patients, chemotherapy causes significant toxicity to multiple organs, including the skeleton, but the underlying mechanisms have yet to be elucidated. Using tumor-free mouse models, which are commonly used to assess direct off-target effects of anti-neoplastic therapies, we found that doxorubicin caused massive bone loss in wild-type mice, a phenotype associated with increased number of osteoclasts, leukopenia, elevated serum levels of danger-associated molecular patterns (DAMPs; e.g. cell-free DNA and ATP) and cytokines (e.g. IL-1β and IL-18). Accordingly, doxorubicin activated the absent in melanoma (AIM2) and NLR family pyrin domain containing 3 (NLRP3) inflammasomes in macrophages and neutrophils, causing inflammatory cell death pyroptosis and NETosis, which correlated with its leukopenic effects. Moreover, the effects of this chemotherapeutic agent on cytokine secretion, cell demise, and bone loss were attenuated to various extent in conditions of AIM2 and/or NLRP3 insufficiency. Thus, we found that inflammasomes are key players in bone loss caused by doxorubicin, a finding that may inspire the development of a tailored adjuvant therapy that preserves the quality of this tissue in patients treated with this class of drugs.






