Stability and Extraction of Vanillin and Coumarin under Subcritical Water Conditions
Abstract
:1. Introduction
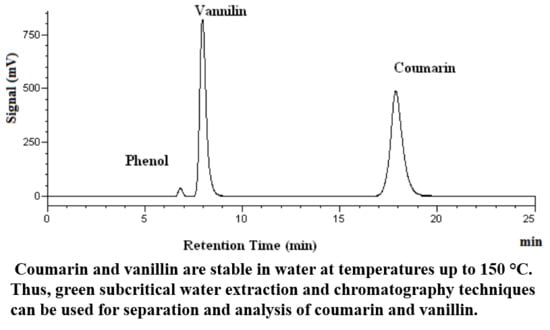
2. Results and Discussions
2.1. Stability of Vanillin and Coumarin in Subcritical Water
2.2. Subcritical Water Extraction
3. Materials and Methods
3.1. Reagents and Materials
3.2. Heating of Organic-Water Mixtures
3.3. Preparation of Standard Solutions
3.4. Sonication Extraction
3.5. Subcritical Water Extraction
3.6. HPLC Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SBWE | Subcritical water extraction |
| HPLC | High-performance liquid chromatography |
| UV | Ultra violet |
References
- Priefert, H.; Rabenhorst, J.; Steinbüchel, A. Biotechnological production of vanillin. Appl. Microbiol. Biotechnol. 2001, 56, 296–314. [Google Scholar] [CrossRef] [PubMed]
- De Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Comparison of headspace-SPME-GC–MS and LC–MS for the detection and quantification of coumarin, vanillin, and ethyl vanillin in vanilla extract products. Food Chem. 2008, 107, 1701–1709. [Google Scholar] [CrossRef]
- De Jager, L.S.; Perfetti, G.A.; Diachenko, G.W. Determination of coumarin, vanillin, and ethyl vanillin in vanilla extract products: Liquid chromatography mass spectrometry method development and validation studies. J. Chromatogr. A 2007, 1145, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Barboni, L.; Caprioli, G.; Papa, F.; Ricciutelli, M.; Sagratini, G.; Vittori, S. HPLC quantification of coumarin in bastard balm (Melittis melissophyllum L., Lamiaceae). Fitoterapia 2011, 82, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Prasad, A.K.; Parmar, V.S.; Ghosh, B.; Saini, N. 7,8-Dihydroxy-4-methylcoumarin induces apoptosis of human lung adenocarcinoma cells by ROS-independent mitochondrial pathway through partial inhibition of ERK/MAPK signaling. FEBS Lett. 2007, 581, 2447–2454. [Google Scholar] [CrossRef] [Green Version]
- Keri, R.S.; Hosamani, K.M.; Shingalapur, R.V.; Hugar, M.H. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Eur. J. Med. Chem. 2010, 45, 2597–2605. [Google Scholar] [CrossRef]
- Sproll, C.; Ruge, W.; Andlauer, C.; Godelmann, R.; Lachenmeier, D.W. HPLC analysis and safety assessment of coumarin in foods. Food Chem. 2008, 109, 462–469. [Google Scholar] [CrossRef]
- Krishna veni, N.; Meyyanathan, S.N.; Aduri, A.R.; Alkeshbhai, S.S.; Elango, K. Analysis of vanillin in food products by high performance thin layer chromatography. J. Adv. Sci. Res. 2013, 4, 48–51. [Google Scholar]
- Yang, Y. Subcritical water chromatography: A green approach to high-temperature liquid chromatography. J. Sep. Sci. 2007, 30, 1131–1140. [Google Scholar] [CrossRef]
- Yang, Y.; Kapalavavi, B. Subcritical water chromatography—An economical and green separation technique. Encycl. Anal. Chem. 2011, 1–23. [Google Scholar] [CrossRef]
- Smith, R. Superheated water chromatography – A green technology for the future. J. Chromatogr. A 2008, 1184, 441–455. [Google Scholar] [CrossRef]
- Doctor, N.; Yang, Y. Separation and Analysis of Aspirin and Metformin HCl Using Green Subcritical Water Chromatography. Molecules 2018, 23, 2258. [Google Scholar] [CrossRef] [Green Version]
- Kapalavavi, B.; Yang, Y.; Marple, R.; Gamsky, C. Separation and analysis of pharmaceuticals in cold drugs using green chromatography. Sep. Purif. Technol. 2016, 158, 308–312. [Google Scholar] [CrossRef]
- Scott, A.F.; Thurbide, K.B.; Quickfall, D. A comparison of hydrocarbon and alkali metal response in the flame ionization detector used in subcritical water chromatography. Can. J. Chem. 2015, 93, 784–789. [Google Scholar] [CrossRef]
- Akay, S.; Odabaşı, M.; Yang, Y.; Kayan, B. Synthesis and evaluation of NA-PHEMAH polymer for use as a new stationary phase in high-temperature liquid chromatography. Sep. Purif. Technol. 2015, 152, 1–6. [Google Scholar] [CrossRef]
- Srisopa, A. Preparation of monodisperse porous poly(glycidylmethacrylate-co-ethylenedimethacrylate) microspheres and their application as stationary phase for superheated water HPLC. Talanta 2016, 147, 358–363. [Google Scholar] [CrossRef]
- Yang, Y.; Kapalavavi, B.; Gujjar, L.; Hadrous, S.; Marple, R.; Gamsky, C. Industrial application of green chromatography - II. Separation and analysis of preservatives in skincare products using subcritical water chromatography. Int. J. Cosmet. Sci. 2012, 34, 466–476. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, X.; Mao, Y.; Zhang, Y.; Liu, J.; Rong, L.; Xu, Z. Retention mechanism of phenolic compounds in subcritical water chromatography. Chem. Res. Chin. Univ. 2015, 31, 103–106. [Google Scholar] [CrossRef]
- Kayan, B.; Akay, S.; Yang, Y. Green Chromatographic Separation of Coumarin and Vanillins Using Subcritical Water as the Mobile Phase. J. Chromatogr. Sci. 2016, 54, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Kapalavavi, B.; Gamsky, C.; Marple, R.; Yang, Y. Separation of sunscreens in skincare creams using greener high?temperature liquid chromatography and subcritical water chromatography. Int. J. Cosmet. Sci. 2011, 34, 169–175. [Google Scholar] [CrossRef]
- Droux, S.; Roy, M.; Felix, G. Green chiral HPLC study of the stability of Chiralcel OD under high temperature liquid chromatography and subcritical water conditions. J. Chromatogr. B 2014, 968, 22–25. [Google Scholar] [CrossRef]
- Yang, Y.; Strickland, Z.; Kapalavavi, B.; Marple, R.; Gamsky, C. Industrial application of green chromatography—I. Separation and analysis of niacinamide in skincare creams using pure water as the mobile phase. Talanta 2011, 84, 169–174. [Google Scholar] [CrossRef]
- Yarita, T.; Aoyagi, Y.; Sasai, H.; Nishigaki, A.; Shibukawa, M. Separation of parabens on a zirconia-based stationary phase in superheated water chromatography. Anal. Sci. 2013, 29, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Kapalavavi, B.; Marple, R.; Gamsky, C.; Yang, Y. Studies on the stability of preservatives under subcritical water conditions. Int. J. Cosmet. Sci. 2015, 37, 306–311. [Google Scholar] [CrossRef]
- Lindquist, E.; Yang, Y. Degradation of benzoic acid and its derivatives in subcritical water. J. Chromatogr. A 2011, 1218, 2146–2152. [Google Scholar] [CrossRef]
- Yang, Y.; Kayan, B.; Bozer, N.; Pate, B.; Baker, C.; Gizir, A.M. Terpene degradation and extraction from basil and oregano leaves using subcritical water. J. Chromatogr. A 2007, 1152, 262–267. [Google Scholar] [CrossRef]
- Fujii, T.; Kawasaki, S.-I. Effects of process parameters on vanillin partition coefficient in water-supercritical CO2 extraction. Fluid Phase Equilibria 2019, 485, 153–157. [Google Scholar] [CrossRef]
- Fujii, T.; Kawasaki, S.-I. Salting-out effects on vanillin extraction by supercritical carbon dioxide from aqueous vanillin solution containing salts. J. Supercrit. Fluids 2019, 152, 104550. [Google Scholar] [CrossRef]
- Jadhav, D.; Rekha, B.N.; Gogate, P.R.; Rathod, V.K. Extraction of vanillin from vanilla pods: A comparison study of conventional soxhlet and ultrasound assisted extraction. J. Food Eng. 2009, 93, 421–426. [Google Scholar] [CrossRef]
- Rodríguez-Jimenes, G.D.C.; Vargas-Garcia, A.; Espinoza-Pérez, D.J.; Salgado-Cervantes, M.A.; Robles-Olvera, V.J.; A García-Alvarado, M. Mass Transfer During Vanilla Pods Solid Liquid Extraction: Effect of Extraction Method. Food Bioprocess Technol. 2012, 6, 2640–2650. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical Water Extraction of Biological Materials. Sep. Purif. Rev. 2016, 46, 21–34. [Google Scholar] [CrossRef]
- Kawamura, F.; Saary, N.S.; Hashim, R.; Sulaiman, O.; Hashida, K.; Otsuka, Y.; Nakamura, M.; Ohara, S. Subcritical Water Extraction of Low-molecular-weight Phenolic Compounds from Oil Palm Biomass. Jpn. Agric. Res. Quarterly: JARQ 2014, 48, 355–362. [Google Scholar] [CrossRef] [Green Version]






| Temperature | 15 min | 60 min | ||
|---|---|---|---|---|
| (°C) | % Recovery | % RSD a | % Recovery | % RSD a |
| 100 | 97 | 5 | 93 | 6 |
| 150 | 103 | 5 | 98 | 2 |
| 200 | 97 | 2 | 99 | 2 |
| 250 | 99 | 1 | 95 | 2 |
| Temperature | 15 min | 60 min | ||
|---|---|---|---|---|
| (°C) | % Recovery | % RSD a | % Recovery | % RSD a |
| 100 | 98 | 6 | 96 | 7 |
| 150 | 101 | 4 | 100 | 7 |
| 200 | 97 | 3 | 91 | 10 |
| 250 | 101 | 5 | 82 | 20 |
| Temperature | Vanillin | Coumarin | ||
|---|---|---|---|---|
| % Recovery | % RSD a | % Recover | % RSD a | |
| 100 °C | 98.4 | 0.6 | 96.9 | 0.7 |
| 150 °C | 104.2 | 0.8 | 102.3 | 0.8 |
| 200 °C | 104 | 0.6 | 102.2 | 0.6 |
| Temperature (°C) | Vanillin in Vanilla Beans | Coumarin in Tonka Beans | ||
|---|---|---|---|---|
| mg/g | % RSD a | mg/g | % RSD a | |
| 100 | 1.07 | 9 | 23.9 | 6 |
| 150 | 1.66 | 3 | 25.2 | 3 |
| 200 | 1.95 | 5 | 36.8 | 9 |
| Sonication | 4.97 | 5 | 102.5 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doctor, N.; Parker, G.; Vang, K.; Smith, M.; Kayan, B.; Yang, Y. Stability and Extraction of Vanillin and Coumarin under Subcritical Water Conditions. Molecules 2020, 25, 1061. https://doi.org/10.3390/molecules25051061
Doctor N, Parker G, Vang K, Smith M, Kayan B, Yang Y. Stability and Extraction of Vanillin and Coumarin under Subcritical Water Conditions. Molecules. 2020; 25(5):1061. https://doi.org/10.3390/molecules25051061
Chicago/Turabian StyleDoctor, Ninad, Grayson Parker, Katie Vang, Melanie Smith, Berkant Kayan, and Yu Yang. 2020. "Stability and Extraction of Vanillin and Coumarin under Subcritical Water Conditions" Molecules 25, no. 5: 1061. https://doi.org/10.3390/molecules25051061