Abstract
Purpose
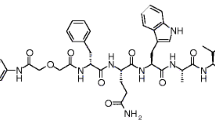
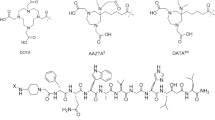
The goal of this work was to develop hydrophilic gastrin-releasing peptide receptor (GRPR)-targeting complexes of the general formula fac-[M(CO)3(L)]+ [M = natRe, 99mTc, 186Re; L: NOTA for 1, NODAGA for 2] conjugated to a powerful GRPR peptide antagonist (DPhe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2) via a 6-aminohexanoic acid linker.
Procedures
Metallated-peptides were prepared employing the [M(OH2)3(CO)3]+ [M = Re, 99mTc, 186Re] precursors. Re-1/2 complexes were characterized with HR-MS. IC50 studies were performed for peptides 1/2 and their respective Re-1/2 complexes in a binding assay utilizing GRPR-expressing human PC-3 prostate cancer cells and [125I]I-Tyr4-BBN as the competing ligand. The 99mTc/186Re-complexes were identified by HPLC co-injection with their Re-analogues. All tracers were challenged in vitro at 37 °C against cysteine/histidine (phosphate-buffered saline 10 mM, pH 7.4) and rat serum. Biodistribution and micro-SPECT/CT imaging of [99mTc]Tc-1/2 and [186Re]Re-2 were performed in PC-3 tumor-bearing ICR SCID mice.
Results
High in vitro receptor affinity (IC50 2–3 nM) was demonstrated for all compounds. The 99mTc/186Re-tracers were found to be hydrophilic (log D7.4 ≤ − 1.35) and highly stable. Biodistribution in PC-3 xenografted mice revealed good tumor uptake (%ID/g at 1 h: 4.3 ± 0.7 for [99mTc]Tc-1, 8.3 ± 0.9 for [99mTc]Tc-2 and 4.2 ± 0.8 for [186Re]Re-2) with moderate retention over 24 h. Rapid renal clearance was observed for [99mTc]Tc-2 and [186Re]Re-2 (> 84 % at 4 h), indicating favorable pharmacokinetics. Micro-SPECT/CT images for the 99mTc-tracers clearly visualized PC-3 tumors in agreement with the biodistribution data and with superior imaging properties found for [99mTc]Tc-2.
Conclusions
[99mTc]Tc-2 shows promise for further development as a GRPR-imaging agent. [186Re]Re-2 demonstrated very similar in vivo behavior to [99mTc]Tc-2, and further studies are therefore justified to explore the theranostic potential of our approach for targeting of GRPR-positive cancers.





Similar content being viewed by others
References
Reubi JC (2003) Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24:389–427
Dash A, Chakraborty S, Pillai MRA, Knapp FFR (2015) Peptide receptor radionuclide therapy: an overview. Cancer Biother Radiopharm 30:47–71
Lee S, Xie J, Chen X (2010) Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev 110:3087–3111
Mansi R, Wang X, Forrer F, Waser B, Cescato R, Graham K, Borkowski S, Reubi JC, Maecke HR (2011) Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging 38:97–107
Dumont RA, Tamma M, Braun F, Borkowski S, Reubi JC, Maecke H, Weber WA, Mansi R (2013) Targeted radiotherapy of prostate cancer with a gastrin-releasing peptide receptor antagonist is effective as monotherapy and in combination with rapamycin. J Nucl Med 54:762–769
Dalm SU, Bakker IL, de Blois E, Doeswijk GN, Konijnenberg MW, Orlandi F, Barbato D, Tedesco M, Maina T, Nock BA, de Jong M (2017) 68Ga/177Lu-NeoBOMB1, a novel radiolabeled GRPR antagonist for theranostic use in oncology. J Nucl Med 58:293–299
Schibli R, La Bella R, Alberto R et al (2000) Influence of the denticity of ligand systems on the in vitro and in vivo behavior of 99mTc(I)-tricarbonyl complexes: a hint for the future functionalization of biomolecules. Bioconjug Chem 11:345–351
Schibli R, Schwarzbach R, Alberto R, Ortner K, Schmalle H, Dumas C, Egli A, Schubiger PA (2002) Steps toward high specific activity labeling of biomolecules for therapeutic application: preparation of precursor [188Re(H2O)3(CO)3]+ and synthesis of tailor-made bifunctional ligand systems. Bioconjug Chem 13:750–756
Schweinsberg C, Maes V, Brans L, Bläuenstein P, Tourwé DA, Schubiger PA, Schibli R, Garayoa EǴ (2008) Novel glycated [99mTc(CO)3]-labeled bombesin analogues for improved targeting of gastrin-releasing peptide receptor-positive tumors. Bioconjug Chem 19:2432–2439
Lane SR, Veerendra B, Rold TL, Sieckman GL, Hoffman TJ, Jurisson SS, Smith CJ (2008) 99mTc(CO)3-DTMA bombesin conjugates having high affinity for the GRP receptor. Nucl Med Biol 35:263–272
Retzloff LB, Heinzke L, Figureoa SD et al (2010) Evaluation of [99mTc-(CO)3-X-Y-bombesin(7-14)NH2] conjugates for targeting gastrin-releasing peptide receptors overexpressed on breast carcinoma. Anticancer Res 30:19–30
Smith CJ, Sieckman GL, Owen NK et al (2003) Radiochemical investigations of gastrin-releasing peptide receptor-specific [99mTc(X)(CO)3-Dpr-Ser-Ser-Ser-Gln-Trp-Ala-Val-Gly- His-Leu-Met-(NH2)] in PC-3, tumor-bearing, rodent models: syntheses, radiolabeling, and in vitro/in vivo studies where Dpr = 2, 3-Diaminopropionic acid and X= H2O or P(CH2OH)3. Cancer Res 63:4082–4088
Makris G, Radford LL, Kuchuk M, Gallazzi F, Jurisson SS, Smith CJ, Hennkens HM (2018) NOTA and NODAGA [99mTc]Tc- and [186Re]Re-tricarbonyl complexes: radiochemistry and first example of a [99mTc]Tc-NODAGA somatostatin receptor-targeting bioconjugate. Bioconjug Chem 29:4040–4049
Makris G, Kuchuk M, Gallazzi F, Jurisson SS, Smith CJ, Hennkens HM (2019) Somatostatin receptor targeting with hydrophilic [99mTc/186Re]Tc/Re-tricarbonyl NODAGA and NOTA complexes. Nucl Med Biol 71:39–46
Llinares M, Devin C, Chaloin O, Azay J, Noel-Artis AM, Bernad N, Fehrentz JA, Martinez J (1999) Syntheses and biological activities of potent bombesin receptor antagonists. J Pept Res 53:275–283
Prasanphanich AF, Nanda PK, Rold TL, Ma L, Lewis MR, Garrison JC, Hoffman TJ, Sieckman GL, Figueroa SD, Smith CJ (2007) [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] targeting vector for positron-emission tomography imaging of gastrin-releasing peptide receptor-expressing tissues. Proc Natl Acad Sci U S A 104:12462–12467
Maresca KP, Marquis JC, Hillier SM, Lu G, Femia FJ, Zimmerman CN, Eckelman WC, Joyal JL, Babich JW (2010) Novel polar single amino acid chelates for technetium-99m tricarbonyl-based radiopharmaceuticals with enhanced renal clearance: application to octreotide. Bioconjug Chem 21:1032–1042
Qiao Z, Xu J, Gonzalez R, Miao Y (2020) Novel [99mTc]-tricarbonyl-NOTA- conjugated lactam-cyclized alpha-MSH peptide with enhanced melanoma uptake and reduced renal uptake. Mol Pharmaceutics https://doi.org/10.1021/acs.molpharmaceut.0c00606
Veerendra B, Sieckman G, Hoffman T et al (2006) Synthesis, radiolabeling and in vitro GRP receptor targeting studies of 99mTc-Triaza-X-BBN[7-14]NH2 (X = Serylserylserine, Glycylglycylglycine, glycylserylglycine, or beta alanine). Synth React Inorganic Met Nano-Met Chem 36:481–491
Cescato R, Mansi R, Nicolas G et al (2011) Bombesin antagonist-based radioligands for translational nuclear imaging of gastrin-releasing peptide receptor-positive tumors. J Nucl Med 52:1970–1978
García Garayoa E, Schweinsberg C, Maes V, Rüegg D, Blanc A, Bläuenstein P, Tourwé DA, Beck-Sickinger AG, Schubiger PA (2007) New [99mTc]bombesin analogues with improved biodistribution for targeting gastrin releasing-peptide receptor-positive tumors. Q J Nucl Med Mol Imaging 51:42–50
García Garayoa E, Schweinsberg C, Maes V et al (2008) Influence of the molecular charge on the biodistribution of bombesin analogues labeled with the [99mTc(CO)3]-core. Bioconjug Chem 19:2409–2416
Brans L, García-Garayoa E, Schweinsberg C, Maes V, Struthers H, Schibli R, Tourwé D (2010) Synthesis and evaluation of bombesin analogues conjugated to two different triazolyl-derived chelators for 99mTc labeling. ChemMedChem 5:1717–1725
Alves S, Correia JDG, Santos I, Veerendra B, Sieckman GL, Hoffman TJ, Rold TL, Figueroa SD, Retzloff L, McCrate J, Prasanphanich A, Smith CJ (2006) Pyrazolyl conjugates of bombesin: a new tridentate ligand framework for the stabilization of fac-[M(CO)3]+ moiety. Nucl Med Biol 33:625–634
Fani M, Del Pozzo L, Abiraj K et al (2011) PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med 52:1110–1118
Acknowledgments
This study was funded by the University of Missouri Research Reactor (Hennkens, internal support) and the US Department of Veterans’ Affairs (Smith, VA MERIT Application 1I01BX003392). We gratefully acknowledge Mid-America Isotopes, Inc. (Ashland, MO) for the generous donation of 99Mo/99mTc generators and the support provided by the VA Biomolecular Imaging Center at the Harry S. Truman VA Hospital and the University of Missouri-Columbia. For technical expertise and contributions, we thank Mary Embree and Daniel Ampong-O’Connor (186Re production), Dr. Fabio Gallazzi (LC-ESI-MS), Dr. Brian P. Mooney (HR-ESI-MS), Susan Rottinghaus (cell culture), Lisa Watkinson and Terry Carmack (biodistribution studies), and Ashley Berendzen (micro-SPECT/CT studies).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Drs. Hennkens and Makris have a patent US Application 62/932,530, “Metal Chelators and Complexes” pending to The Curators of the University of Missouri. All other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 232 kb)
Rights and permissions
About this article
Cite this article
Makris, G., Bandari, R.P., Kuchuk, M. et al. Development and Preclinical Evaluation of 99mTc- and 186Re-Labeled NOTA and NODAGA Bioconjugates Demonstrating Matched Pair Targeting of GRPR-Expressing Tumors. Mol Imaging Biol 23, 52–61 (2021). https://doi.org/10.1007/s11307-020-01537-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11307-020-01537-1