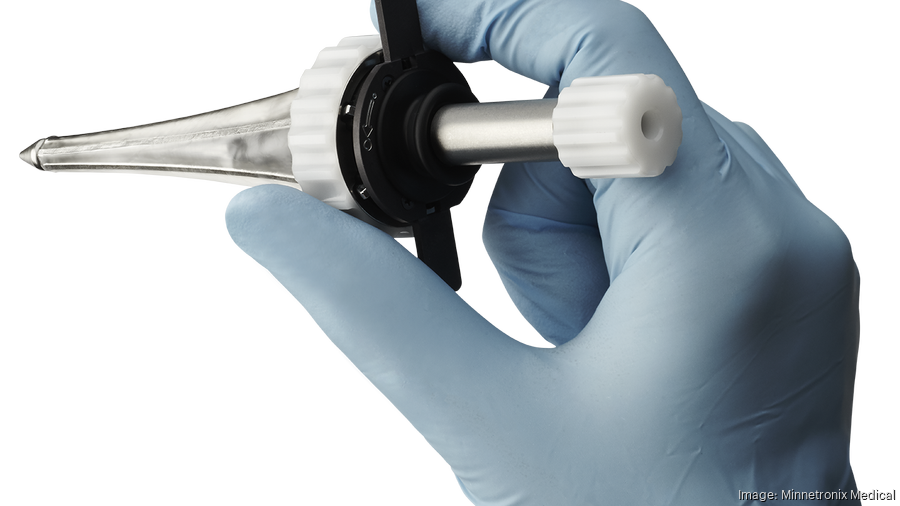
Minnetronix Medical had been making other people's medical devices since 1996, but it had never brought its own product to the market. Until 2020, that is.
Last year in September, the medical device contract manufacturer received FDA clearance for the MindsEye Port, a minimally invasive port used in surgeries that treat strokes, cancer and other conditions. It's the first port of its kind and the first product Minnetronix has innovated on that can be inserted in the body.
Until then, Minnetronix had been focused on making medical devices for other companies that outsourced part of their development. The St. Paul-based company has 400 employees, including over 150 engineers and scientists. Coming up with an original device had been a goal of Minnetronix for at least a decade, and years of working with other medical device companies had helped it identify unaddressed needs, CEO Jeremy Maniak said.
"Our first-hand experience, and the finished products themselves, enable our team to bring unique knowledge and end-to-end experience to our customers," he said.
The MindsEye Port is designed to reduce the risk of secondary injuries caused by brain surgery, Maniak said. The port can shrink or grow once it's inserted into the patient's head, which can allow surgeons to reach specific areas in the brain.
"This is next-generation deep brain access technology," Mario Zuccarello, a University of Cincinnati Medical Centre neurosurgery professor, said in a statement when the product received FDA clearance.
The expensive and onerous process of getting FDA clearance was entirely internally funded and directed by Minnetronix, Maniak said.
And Minnetronix isn't done innovating. It's working on a Neurapheresis Therapy device that decontaminates cerebrospinal fluid after a hemorrhagic stroke. That device has been years in the making, and Minnetronix expects to release the clinical results of a feasibility trial and submit the device for FDA clearance later this year, Maniak said. Other projects are also in the works.
"We intend to continue to identify unmet needs in niche markets that allow us to deliver additional innovative technologies to medical device companies," Maniak said.
In May 2020, Minnetronix was tapped as one of eight U.S. manufacturers selected by NASA’s Jet Propulsion Laboratory to make its VITAL, a ventilator that only required one-seventh of the parts needed in a traditional ventilator, to help Covid-19 patients in the early days of the pandemic.
Minnetronix Medical
CEO: Jeremy Maniak
Headquarters: St. Paul
Employees: 400
Year founded: 1996
Business: Medical device manufacturer
Web: minnetronixmedical.com