Review of immune checkpoint inhibitor and CAR T-cell therapy adverse effects and treatments.
Takeaways:
- The U.S. Food and Drug Administration has approved several immunotherapy agents to treat cancer, including immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapy.
- Adverse effects of immunotherapy can affect multiple systems, including neurologic, musculoskeletal, cardiovascular, and respiratory.
- Nurses have a critical role to play in caring for and monitoring patients receiving cancer immunotherapy.
CNE 1.5 contact Hours
Learning Objectives
- Describe how immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapy work.
- Discuss adverse effects related to immune checkpoint inhibitors.
- Discuss adverse effects related to CAR T-cell therapy.
The authors and planners of this CNE activity have disclosed no relevant financial relationships with any commercial companies pertaining to this activity. See the last page of the article to learn how to earn CNE credit.
Expiration: 9/1/23
Because cancer treatment (surgery, radiation, and chemotherapy) frequently fails, researchers began asking why patients’ immune systems don’t respond to cancer cells like they do to bacteria and viruses. Under normal conditions, T lymphocytes (T-cells) bind to a tumor cell via programmed cell death protein 1 (PD-1) and programmed death ligand 1 (PD-L1) receptors and then send signals that recruit antigen-presenting cells (APCs) that attach to the T-cells via the cytotoxic T lymphocyte antigen-4 (CTLA-4) binding arm. Next, a series of cytokines (such as gamma interferon, interleukin 12 and B7) work to kill the T-cell and the tumor cell through apoptosis (cell death). Cancer tumor cells, however, block T-cells from binding to dendritic cells, allowing the cancer to escape detection by the patient’s immune system.
The U.S. Food and Drug Administration (FDA) has approved several immunotherapy agents to treat cancer. They help the immune system recognize tumor cells, prevent immune system tolerance of tumor cells, and manipulate translational proteins in the tumor microenvironment. However, these drugs come with accompanying toxicities (immune-related adverse effects). This article focuses on two recently approved treatments—immune checkpoint inhibitors and chimeric antigen receptor (CAR) T-cell therapy—their immune-related adverse effects, and treatment options. By understanding how these treatments work and their possible adverse effects, nurses can better educate patients and intervene quickly when necessary.
Immune checkpoint inhibitors
Immune checkpoint inhibitors (CTLA-4, PD-1, and PD-L1) have emerged as potential targets in the development of new cancer drugs.
CTLA-4 inhibitors
Immune tolerance is the process described by Schwartz as an “anticipatory organ” that has learned to recognize self from nonself and prevent self-destruction through a series of mechanisms that include T-cells, APCs, cellular receptors, and mediators. CTLA-4 is key in this process.
CTLA-4 is a receptor that competes for binding with other receptors to regulate T-cell proliferation and inflammatory cytokine production. Intact CTLA-4 dampens inflammation and preserves immune tolerance, keeping the body’s immune response in check. Tumors recognize the advantage of stimulating T-cells to express many CTLA-4 antigens on their surface and inhibit APCs from recognizing and killing tumor cells. Anti-CTLA-4 monoclonal antibodies block CTLA-4, so that T-cells can then destroy tumor cells.
Ipilimumab is the only anti-CTLA-4 monoclonal antibody FDA-approved to treat cancer; it’s indicated for advanced melanoma and renal cell and colon cancer. It is also used for liver carcinoma, small cell lung cancer, and esophageal cancer with orphan drug status. Ipilimumab is used as an adjunct to alkylating drugs like dacarbazine for melanoma and docetaxel for renal cell cancer or in combination with an anti-PD-1 drug. It’s administered I.V. in a monitored setting.
PD-1 and PD-L1 inhibitors
PD-1 in healthy tissue promotes self-tolerance; when deficient, it leads to autoimmune diseases. PD-L1 rarely occurs in healthy tissue, but is present on cancer cells. It’s induced by the production of the chemokine interferon gamma by T-cells recruited to the tumor microenvironment. Interferon gamma initiates inflammation and PD-L1 expression. PD-1 on the T-cell binds to PD-L1 on the tumor surface, permanently switching off T-cell recognition and preventing T-cells from inducing tumor cell death and causing immune fatigue. Monoclonal antibodies have been developed that bind to PD-1 or PD-L1 to modulate immune suppression induced by the tumor in its microenvironment and resuscitate the patient’s immune system.
Immune checkpoint inhibitor–related adverse effects
Immune checkpoint inhibitor disruption of the tolerance of self vs. nonself leads to multiple organ system alterations. Before initiating therapy, providers should obtain a detailed patient baseline history, perform a comprehensive physical, and document findings in the electronic health record.
Baseline lab tests include a complete metabolic panel, liver enzymes, complete blood count, thyroid levels, cortisol levels, baseline scans, ECG, weight, and body mass index. As indicated by pre-existing conditions, echocardiograms, pulmonary function tests, pancreatic enzymes, and musculoskeletal exams also should be performed. Monitoring the findings, obtaining detailed histories at each clinic visit, and testing per patient status or clinical protocol can help identify adverse signs and symptoms for grading that determines continuation or delay of the immune checkpoint inhibitor and the need for adjunctive treatments to ameliorate its side effects.
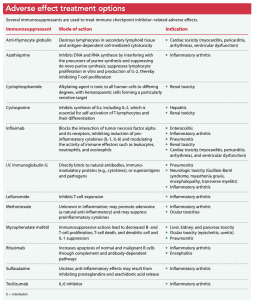
Almost all adverse effects require treatment with immunosuppression. Glucocorticoid steroids are the primary drug of choice; however, other immunosuppressant agents may be used to regulate an overwhelming inflammatory response caused by immune checkpoint inhibitors. (See Adverse effect treatment options.)
Organizational protocols are used for grading adverse effects, choosing treatment strategies, and determining whether immunotherapy will continue.
Common adverse effects
Common immune checkpoint inhibitor–related adverse effects occur most frequently within the integument, endocrine, and GI systems, although less common effects can occur as well.
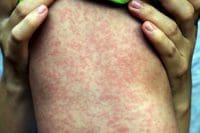
Integument. Skin rashes, vitiligo, and pruritis occur in more than 30% of patients receiving immune checkpoint inhibitor therapy. Integument signs and symptoms generally manifest early in ipilimumab and PD-1 inhibitor treatment. Rashes and pruritus usually can be managed with topical glucocorticoid steroids and don’t require immunotherapy suspension. However, some patients who experience refractory pruritus or Stevens-Johnson syndrome (toxic epidermal necrolysis) may require an immediate dermatology consultation and immunotherapy discontinuation.
Endocrine. The endocrine system is particularly vulnerable to CTLA-4 checkpoint inhibition but toxicities also can be seen in PD-1 and PD-L1 therapy when combined with ipilimumab. Patients show evidence of adverse effects related to ipilimumab typically after the sixth or seventh week of treatment. Time to onset of endocrinopathies caused by pembrolizumab is 10 weeks and 11 weeks for nivolumab. Median onset can be between 1 to 5 months depending on the drug. Endocrinopathies frequently are permanent, and patients require lifetime hormone replacement therapy.
Endocrine adverse effects include autoimmune diabetes, thyroiditis, adrenal insufficiency, and hypophysitis. The decision to continue, hold, or discontinue immunotherapy after an endocrine toxicity diagnosis depends on symptom severity, treatment response, and organization protocols and guidelines.
Autoimmune diabetes presents classically with polyuria, polydipsia, polyphagia, weight loss, and fasting glucose > 126 mg/dL. Autoimmune diabetes diagnosis requires immediate referral to an endocrinologist and may result in discontinuation of immune checkpoint inhibitor therapy; if the diabetes can be controlled, therapy can be resumed.
Asymptomatic thyroiditis can be identified and monitored via laboratory test surveillance. However, if a patient presents with clinical symptoms of hyperthyroidism (with palpitations, increased stool frequency, heat intolerance, sweating, and weight loss) or hypothyroidism (with asthenia, constipation, dry skin, hair loss, and weight gain) treatment is initiated as it would be for patients not receiving immune checkpoint inhibition therapy.
Life-threatening adrenal insufficiency can occur with or without central hypophysitis. Arresting this adverse effect requires early diagnosis and treatment. Typically, patients experience overwhelming fatigue, fever, visual changes, myalgias, weakness, nausea, vomiting, dizziness, and fainting. Their blood pressure is low, heart rate is elevated, and laboratory tests may show low sodium and elevated potassium. Diagnosing central hypophysitis requires hypothalamic hormone (adrenocorticotrophic hormone, follicle-stimulating hormone, luteinizing hormone, prolactin, anti-diuretic hormone, oxytocin, testosterone) testing; low levels suggest hypophysitis. Generally, adrenal insufficiency, crisis, or hypophysitis triggers immune checkpoint inhibitor therapy discontinuation.
GI. Enterocolitis related to immune checkpoint inhibitor therapy generally occurs 6 to 8 weeks after initiating treatment and presents as watery diarrhea, abdominal pain and cramping, nocturnal defecation, tenesmus, defecation urgency, blood and mucus in stools, and fever. The incidence of colitis is high in patients receiving ipilimumab and significantly higher when ipilimumab is administered in combination with nivolumab. Dehydration, weight loss, and electrolyte disturbances may occur, requiring hospitalization for supportive care. Infliximab frequently is used to treat severe or glucocorticoid-resistant symptoms.
Hepatitis and pancreatitis may occur subacutely with abnormal liver or pancreatic enzyme levels. Treatment includes glucocorticoids or mycophenolate mofetil. Infliximab is not a treatment of choice for hepatitis or pancreatitis.
Less common adverse effects
Less common adverse effects of immune checkpoint inhibitor therapy occur in the ocular, cardiovascular, pulmonary, neurologic, and musculoskeletal systems.
Ocular. Patients experiencing ocular toxicities may present with uvea or sclera inflammation. Commonly reported symptoms include decreased visual acuity (blurred vision), trouble distinguishing colors, blind spots, light sensitivity, and eye pain. Clinically, patients may have eyelid swelling, proptosis, or red or purple discoloration of the eye. Adverse effects reported to the FDA suggest more ocular toxicities are associated with PD-1 and PD-L1 inhibitors.
Referral to ophthalmology can confirm diagnosis and determine the degree of inflammation. Mild inflammation typically can be treated with artificial tears; more significant effects may require topical and systemic corticosteroids and immunotherapy delay or discontinuation.
Neurologic. The range of neurologic adverse effects includes peripheral neuropathy, Guillain-Barré syndrome (GBS), myasthenia gravis, aseptic meningitis, encephalopathy, and transverse myelitis. Immune checkpoint inhibitor therapy may be suspended depending on the severity of peripheral neuropathy and if a patient experiences GBS; therapy is permanently discontinued when a patient experiences aseptic meningitis, myasthenia gravis, and transverse myelitis.
GBS presents with symmetrical muscle weakness, decreased or absent deep tendon reflexes, and thigh and back pain. Some patients may also experience oculomotor, bulbar, facial, respiratory, or autonomic nerve dysfunction. Treatment includes critical care observation for airway management, autonomic dysfunction, and administration of corticosteroids, I.V. immunoglobulin, or plasmapheresis exchange.
Myasthenia gravis presents with progressive or fluctuating muscle weakness (generally proximal to distal), ptosis, abnormal extraocular movements, double vision, dysphagia, and facial muscle weakness; it also can be associated with myositis, myocarditis, and pneumonitis. Treatment includes pyridostigmine 30 mg three times a day orally, gradually increased to a maximum dose of 120 mg four times a day as tolerated. Depending on the patient’s symptoms, corticosteroids may be ordered; severe cases may require plasmapheresis and I.V. immunoglobulin.
Aseptic meningitis presents with sudden fever, severe headache, nausea or vomiting, double vision, drowsiness, sensitivity to bright light, and neck stiffness. Encephalitis can be characterized by fever, seizures, behavior changes, confusion, and disorientation. Before treatment can be prescribed, infectious causes (which require appropriate antibacterial, antifungal, or antiviral treatment) must be ruled out via lumbar puncture and lab tests. In the absence of infection, treatment includes corticosteroids; for encephalitis, rituximab may be ordered. Continuing immune checkpoint inhibitor therapy will depend on the toxicity level.
Transverse myelitis (spinal cord inflammation) presents as rapidly progressing weakness in the legs or arms. Pain and sensory alteration as well as constipation, frequent or difficult voiding, incontinence, or partial or complete extremity paralysis may occur. Spinal magnetic resonance imaging, lumbar puncture, and blood tests will rule out infection and detect antibodies associated with immune causes. Treatment includes high-dose corticosteroids, I.V. immunoglobulin, and plasmapheresis.
Cardiovascular. Pericarditis, myocarditis, arrhythmias, and impaired ventricular function are reported more frequently in patients receiving combination ipilimumab and nivolumab than in those receiving other immune checkpoint inhibitor therapies. These high-grade adverse effects require immediate treatment with corticosteroids and I.V. immunoglobulin and immunotherapy discontinuation.
Pulmonary. The most common pulmonary adverse effect is pneumonitis, which occurs most frequently in patients receiving PD-1 inhibitors. Patients with pneumonitis experience dyspnea at rest, cough, decreased oxygen concentration, and mental status changes secondary to hypoxia.
If pulmonary problems occur, immune checkpoint therapy should be held and other symptom causes excluded. Differential diagnoses can include a viral or bacterial respiratory tract infection, pulmonary embolism, abscess, or pneumothorax. Diagnostic tests include a chest computed tomography scan, sputum sample, or nasopharyngeal wash or swab to rule out infectious causes.
If mild symptoms and defects seen on imaging resolve, immunotherapy can be restarted. Moderate to severe symptoms may require hospitalization for support, pulmonary consultation, and treatment with oral or I.V. glucocorticosteroids. Restarting immunotherapy for moderate to severe symptoms depends on arresting symptoms, treating infections, and imaging defect resolution without steroids.
Musculoskeletal. The incidence of inflammatory arthritis and myositis in immune checkpoint inhibitor clinical trials ranges from 1% to 43%. Myositis (joint pain, stiffness, and swelling) is associated with inflammatory arthritis. Musculoskeletal adverse effects can occur with all immune checkpoint inhibitor therapies, but a rare life-threatening myositis is more commonly attributed to PD-1 and PD-L1 therapies.
Glucocorticoids are first-line treatment. For patients whose symptoms don’t respond to glucocorticoids and immunotherapy discontinuation, other options include antitumor necrosis factor alpha drugs and interleukin 6 inhibitors, as well as disease-modifying antirheumatic drugs (such as methotrexate, minocycline, hydroxychloroquine, sulfasalazine, or azathioprine) in place of traditional inflammatory arthritis drugs.
Renal. Rare adverse effects can cause renal failure and acute kidney injury resulting from interstitial nephritis, which typically occurs 6 to 12 weeks after initiating ipilimumab therapy and 3 to 12 months after initiating nivolumab and pembrolizumab therapy.
A one-and-a-half to two times increase of creatinine from baseline may be the first signal of renal toxicity. Guidelines suggest assessing for and discontinuing any concomitant renal toxic drugs (for example, nonsteroidal anti-inflammatories and diuretics) and performing close surveillance. If creatinine rises two to three times from baseline, the provider should hold immunotherapy and order corticosteroids. Creatinine levels greater than four times from baseline are severe; immunotherapy, steroids, and other immunosuppressants (such as azathioprine, monthly cyclophosphamide, cyclosporine, infliximab, or mycophenolate) should be discontinued.
In addition to being aware of these adverse effects, keeping the possibility of pseudoprogression in mind is important when caring for patients who are receiving immune checkpoint inhibitors.
CAR T-cell therapy
CAR T-cell therapy treats refractory, relapsed B-cell malignancies by using the patient’s T-cells re-engineered via a harmless virus carrying the genetic material to recognize cancer-specific antigens for targeted cell killing. Patients who respond to this therapy have profound and enduring remissions. Research of CAR T-cell therapy in multiple myeloma, leukemia, lymphoma, and solid tumors continues with some early reported successes.
CAR T-cell therapy begins with obtaining T-cells from the patient using leukapheresis. 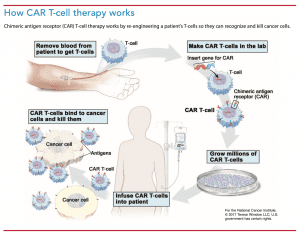 Large-bore venous access is required, and many patients require a central line. Collected T-cells undergo engineering and expansion in centers scrutinized by the FDA Center for Biologics Evaluation and Research.
Large-bore venous access is required, and many patients require a central line. Collected T-cells undergo engineering and expansion in centers scrutinized by the FDA Center for Biologics Evaluation and Research.
Before receiving engineered cells, patients undergo lymphodepleting chemotherapy to make space for the CAR T-cells and kill any residual cancer. Typical chemotherapy agents used include cyclophosphamide and fludarabine. After completing chemotherapy, patients receive the engineered cells. (See How CAR T-cell therapy works.)
Two CAR T-cell products, tisagenlecleucel (Kymriah) and axicabtagene ciloleucel (Yescarta), received approval through the FDA risk evaluation and mitigation strategy to treat B-cell lymphomas and acute lymphoblastic leukemia. The cost of these therapies is around $500,000 per treatment.
Adverse effects
CAR T-cell therapy toxicities affect all organ systems. (See CAR T-cell organ system toxicities.) Cytokine-release syndrome (CRS) is a common life-threatening adverse effect. As re-engineered T-cells move into the circulation, inflammatory cytokines (cell messengers) release pyrogens, vasodilators, and prostaglandins, which leads to clinical manifestations of fever, myalgias, rigor, hypotension, capillary leak, coagulopathies, and hypoxia. Patients require intensive care monitoring and ventilator support, and end-organ failure is common. Researchers’ discovery that a massive release of interleukin 6 occurs during CRS led to treatment with tocilizumab, an anti‐interleukin‐6 receptor antibody. If tocilizumab isn’t effective, corticosteroids must be added, potentially jeopardizing CAR T-cell proliferation; however, this effect hasn’t been proven.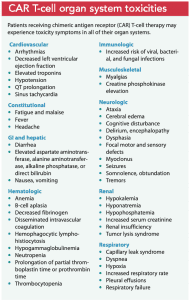
The second most common adverse reaction to CAR T-cell therapy is immune effector cell-associated neurotoxicity syndrome (ICANS), which presents with aphasia and confusion and can progress to loss of consciousness, coma, and seizures. ICANS frequently occurs in conjunction with CRS but is more likely to occur after CRS treatment. ICANS treatment is supportive with corticosteroids, airway management, fall precautions, and one-to-one nursing care. Research into drugs that block the inflammatory interleukin 1 suggests its use may help in severe ICANS cases.
Nursing implications
Nurses have a pivotal and strategic role to play in immunotherapy. They facilitate interdisciplinary collaboration to educate patients and their loved ones about treatment. When nurses understand each therapy’s symptom pathophysiology, they can anticipate side effects, offer anticipatory guidance, and support patients and their families. Nurses also can diligently assess for immune-mediated adverse effects and communicate early with the team to initiate treatment and avoid serious complications. When nurses are familiar with a patient’s psychosocial and spiritual factors, they can help explore palliative care and nonpharmacologic interventions such as reiki, acupunture, and massage. Throughout treatment, nurses, as active members of the healthcare team, can help patients set reasonable expectations and clarify goals.
Side effects can occur early in treatment or present months after therapy ends, making it essential that at discharge patients and their caregivers understand what to look for and to call providers as soon as symptoms appear. Nurses can reassure patients that side effects don’t mean immunotherapy must stop. In most cases, if effects are managed early, treatment can continue.
Most organizations require that patients receiving CAR T-cell therapy have a full-time caregiver. Neurologic side effects of these drugs can be life-threatening, and caregivers who know a patient’s baseline neurologic status can call for help early.
Timely intervention
Providers and researchers are still learning about immunotherapy to treat cancer. Nurses administering these agents should be familiar with their mechanisms of action and adverse effects. Timely intervention can save a patient’s life and ensure treatment continuation.
Victoria L. Anderson is a senior nurse practitioner in the Laboratory of Clinical Immunology and Microbiology, Immunopathogenesis Section at the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH) in Bethesda, Maryland. Leslie Smith is a clinical nurse specialist in oncology, blood and marrow transplant at the NIH Clinical Center Nursing Department in Bethesda, Maryland.
References
Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-68.
Browne BJ. Azathioprine. In: Kaplan B, Burckart GJ, Lakkis FG, eds. Immunotherapy in Transplantation: Principles and Practice. Hoboken, NJ: Wiley; 2012.
Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019;34:45-55.
Buchbinder E, Hodi FS. Cytotoxic T lymphocyte antigen-4 and immune checkpoint blockade. J Clin Invest. 2015;125(9):3377-83.
Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Invest. 2015;125(9):3384-91.
Croxtall JD. Rituximab as first-line maintenance therapy following rituximab-containing therapy for follicular lymphoma. Drugs. 2011;71(7):885-95.
Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: Strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;6:51-71.
Eldridge L. Pseudo-progression with cancer treatment. Verywell Health. August 31, 2019. verywellhealth.com/pseudoprogression-with-cancer-treatment-4692751
Friedman CF, Snyder A. Atypical autoimmune adverse effects with checkpoint blockade therapies. Ann Oncol. 2017;28(2):206-7.
González-Rodríguez E, Rodríguez-Abreu D. Immune checkpoint inhibitors: Review and management of endocrine adverse events. Oncologist. 2016;21(7):804-16.
Izzedine H, Mateus C, Boutros C, et al. Renal effects of immune checkpoint inhibitors. Nephrol Dial Transplant. 2017;32(6):936-42.
Jia W, Gao Q, Han A, Zhu H, Yu J. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med. 2019;16(4):655-70.
Lamprecht M, Dansereau C. CAR T-cell therapy: Update on the state of the science. Clin J Oncol Nurs. 2019;23(2):6-12.
Marks P. The FDA’s regulatory framework for chimeric antigen receptor-T cell therapies. Clin Transl Sci. 2019;12(5):428-30.
Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur J Cancer. 2016;54:139-48.
Möhn N, Beutel G, Gutzmer R, Ivany P, Satzger I, Skripuletz T. Neurological immune related adverse events associated with nivolumab, ipilimumab, and pembrolizumab therapy—Review of the literature and future outlook. J Clin Med. 2019;8(11):1777.
National Comprehensive Cancer Network. NCCN Guidelines Version 2.2019: Management of Immunotherapy-Related Toxicities. NCCN.org. nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf
National Institute of Neurological Disorders and Stroke. Meningitis and encephalitis fact sheet. March 3, 2020. ninds.nih.gov/Disorders/Patient-Caregiver-Education/Fact-Sheets/Meningitis-and-Encephalitis-Fact-Sheet
National Institute of Neurological Disorders and Stroke. Transverse myelitis fact sheet. March 17, 2020. ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/transverse-myelitis-fact-sheet
Neelapu SS. Managing toxicities of CAR T-cell therapy. Hematol Oncol. 2019;37(suppl 1):48-52.
Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158-68.
Sadelain M. CAR therapy: The CD19 paradigm. J Clin Invest. 2015;125(9):3392-400.
Schwartz RH. Historical overview of immunological tolerance. Cold Spring Harb Perspect Biol. 2012;4(4):a006908.
Siddiqui S, Cox J, Herzig R, Palaniyandi S, Hildebrandt GC, Munker R. Anti-thymocyte globulin in haematology: Recent developments. Indian J Med Res. 2019;150(3):221-7.
Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51-60.
Wiseman AC. Immunosuppressive medications. Clin J Am Soc Nephrol. 2016;11(2):332-43.
Yang Y. Cancer immunotherapy: Harnessing the immune system to battle cancer. J Clin Invest. 2015;125(9):3335-7.














4 Comments. Leave new
This was a great article, but I am unable to find the link to download and take the test. When checking the other articles, the download link for the test was at the beginning of the article. Thank you for any assistance you can give me.
Beautiful article on adverse events from immunotherapy, symptom management and educational interventions. Useful for the nurse working in the oncology field
Bellissimo articolo sugli eventi avversi da immunoterapia, sulla gestione dei sintomi e sugli interventi educazionali. Utile per l’infermiere che lavora in ambito oncologico
This was an excellent educational course.