-
PDF
- Split View
-
Views
-
Cite
Cite
Eileen P Scully, Grant Schumock, Martina Fu, Guido Massaccesi, John Muschelli, Joshua Betz, Eili Y Klein, Natalie E West, Matthew Robinson, Brian T Garibaldi, Karen Bandeen-Roche, Scott Zeger, Sabra L Klein, Amita Gupta, for the JH-CROWN registry team, Sex and Gender Differences in Testing, Hospital Admission, Clinical Presentation, and Drivers of Severe Outcomes From COVID-19, Open Forum Infectious Diseases, Volume 8, Issue 9, September 2021, ofab448, https://doi.org/10.1093/ofid/ofab448
Close - Share Icon Share
Abstract
Males experience increased severity of illness and mortality from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) compared with females, but the mechanisms of male susceptibility are unclear.
We performed a retrospective cohort analysis of SARS-CoV-2 testing and admission data at 5 hospitals in the Maryland/Washington DC area. Using age-stratified logistic regression models, we quantified the impact of male sex on the risk of the composite outcome of severe disease or death (World Health Organization score 5–8) and tested the impact of demographics, comorbidities, health behaviors, and laboratory inflammatory markers on the sex effect.
Among 213 175 SARS-CoV-2 tests, despite similar positivity rates, males in age strata between 18 and 74 years were more frequently hospitalized. For the 2626 hospitalized individuals, clinical inflammatory markers (interleukin-6, C-reactive protein, ferritin, absolute lymphocyte count, and neutrophil:lymphocyte ratio) were more favorable for females than males (P < .001). Among 18–49-year-olds, male sex carried a higher risk of severe outcomes, both early (odds ratio [OR], 3.01; 95% CI, 1.75 to 5.18) and at peak illness during hospitalization (OR, 2.58; 95% CI, 1.78 to 3.74). Despite multiple differences in demographics, presentation features, comorbidities, and health behaviors, these variables did not change the association of male sex with severe disease. Only clinical inflammatory marker values modified the sex effect, reducing the OR for severe outcomes in males aged 18–49 years to 1.81 (95% CI, 1.00 to 3.26) early and 1.39 (95% CI, 0.93 to 2.08) at peak illness.
Higher inflammatory laboratory test values were associated with increased risk of severe coronavirus disease 2019 for males. A sex-specific inflammatory response to SARS-CoV-2 infection may underlie the sex differences in outcomes.
Heterogeneity in the outcomes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has been a hallmark of the coronavirus disease 2019 (COVID-19) pandemic. Early reports from Wuhan, China [1, 2], and European countries [3] showed higher rates of hospitalization, intensive care unit (ICU) admission, and mortality in males. Ongoing surveillance [4] and meta-analyses of >3 million cases [5] demonstrate a similar proportion of COVID-19 cases between the sexes, but adult males are almost 3 times more likely to be admitted to ICUs and twice as likely to die as females. Despite a year of the SARS-CoV-2 pandemic, the underlying drivers of sex differential outcomes remain poorly defined [6].
Behaviors, occupations, and societal and cultural norms that also impact the probability of access to testing and health care [7] may account for some differences in exposure to SARS-CoV-2. Gendered associations with health behaviors, including higher tobacco and alcohol use among males [8, 9], also have potential implications for COVID-19 pathogenesis [10, 11]. However, these factors vary across global regions, and the cross-cultural emergence of a male excess of severe outcomes suggests that there may be a direct impact of biological sex on susceptibility to SARS-CoV-2 infection and the pathogenesis of disease [6].
Sex is a modifier of the response to a number of viruses [12, 13], including influenza [14], HIV [15], hepatitis C [16], and SARS-CoV [17, 18]. Immune responses are directly modified by sex steroids [19] and by sex-specific patterns of gene expression [20], which collectively contribute to differences in disease outcomes [12]. The impact of sex steroids differs across age strata, and age-specific patterns of sex differential susceptibility provide mechanistic insights [21, 22]. In COVID-19, sex-specific features of the immune response have been identified, including lower interleukin (IL)-6 levels in females [23], higher levels of C-reactive protein (CRP) in males [24], more durable T-cell responses in females [25], and an association of female sex with lower antibody responses [26]. While these differences support the sex specificity of immune responses, they do not elucidate cause vs consequence of the differences in disease severity.
Despite the many potential mechanisms and evidence of sex differential susceptibility, few large studies have integrated analysis of demographic data, clinical features, and inflammatory markers with sex and age. We leveraged a database of >200 000 SARS-CoV-2 tests and detailed patient-level data from >2600 individuals hospitalized with COVID-19 to define differences between males and females in testing, admission, baseline comorbidities and health behaviors, medication use, laboratory markers, and outcomes. These data were used to estimate the factors with the greatest impact on age and sex differential COVID-19 disease severity.
METHODS
Study Design and Participants
This is a retrospective cohort analysis of patients tested for SARS COV-2 and treated between March 11, 2020, and October 31, 2020, at the Johns Hopkins Medicine health care system locations in the Maryland and Washington DC region (design and inclusion as described previously [27]). This health system is a network of referring clinics and 5 hospitals with 2513 beds including 354 intensive care unit beds serving a population of ~7 million. Data were managed in the JH-CROWN registry: the COVID-19 PMAP Registry, utilizing the Johns Hopkins Precision Medicine Analytics Platform to extract electronic health records [28].
Patient Consent
The institutional review boards of the participating hospitals approved this study as minimal risk and waived consent requirements.
Definitions and Outcome Measures
We stratified data by sex and age: 18–49 (reproductive age), 50–64, 65–74, and 75 years or older. Testing data included both symptomatic and asymptomatic testing, presenting raw numbers and percent positive, including only the first positive test. Natural language processing was used on the data from the admitted cohort to identify presenting symptoms [27]. Initial laboratory values are reported as the median value of results between –48 and +48 hours of hospital admission. Peak and nadir values are from the full course of admission. Labs analyzed included those postulated to have importance in SARS-CoV-2: D-dimer, ferritin, CRP, IL-6, absolute lymphocyte count (ALC), and neutrophil:lymphocyte ratio (NLR). Baseline health status labs in models included initial albumin, hemoglobin, estimated glomerular filtration rate (eGFR), and alanine aminotransferase (ALT).
Primary outcomes were defined using the World Health Organization (WHO) COVID-19 disease severity scale with an ordinal value between 1 and 8 [29]. In the hospitalized cohort, we defined mild/moderate disease as 3–4, severe disease as a 5–7, and the composite outcome of severe disease or death as 5–8. Multiple comorbid condition burden was assessed using the 17-item modified Charlson Comorbidity Index (CCI). Individual comorbidities were extracted from the medical records. Medications were recorded as used if there was an order and at least 1 recorded time of administration.
Statistical Analyses
Raw numbers and percentages of SARS-CoV-2-positive tests and hospital admission among persons with positive tests were tested for difference by sex with the chi-square test. Descriptive cohort characteristics were compared using the chi-square or Fisher exact test, as noted in the legends. Continuous variables were compared with the t test or, for non-Gaussian distributions, with the Wilcoxon rank-sum test, as indicated in the legends.
We developed a logistic regression model to estimate age-specific odds ratios (ORs) comparing male/female incidence of severe disease/death vs mild/moderate disease among persons hospitalized. Final disposition status was available for all except for 1 of the individuals included in the cohort (>99.9%). The base model included hospital of admission and race/ethnicity and was stratified by age. We separately modeled 2 outcomes: severe disease/death at 24 hours after admission (“24-hour” model) and at the most severe point during the hospital admission (“peak status” model). To determine the effect of potential mediating variables on the age-specific sex OR, we divided variables into 6 blocks. Each block was separately added to assess for modification of the sex effect in each age stratum. Variable blocks were defined as follows: block 1 – BMI and admission source (nursing home vs other); block 2 – comorbidities using diagnosis codes that identified asthma, hypertension (complicated and uncomplicated), diabetes, chronic kidney disease, cardiovascular disease, COPD, and immune suppression; block 3 – health behaviors including smoking status identified as current, former, never, and alcohol use; block 4 – presenting vital signs including respiratory rate (RR), fever, SpO2:FiO2 ratio, pulse (median over the first 24 hours of presentation); block 5 – general status labs on presentation: albumin, ALT, hemoglobin, eGFR; block 6 – inflammatory labs: median initial value of ferritin, CRP, D-dimer, ALC, NLR. CRP, ferritin, ALC, and ALT were log-transformed in the models. IL-6 was excluded from models as >50% of values were missing and would have required imputation. Missing values for other variables were imputed before model fitting using Multiple Imputation by Chain Equations (MICE).[30] We report point estimates with 95% confidence intervals for the age-specific sex effects and the block-adjusted age-specific sex effects. The change in the age-specific sex OR associated with block addition is reported as the change in the log OR with a 95% CI determined by bootstrapping. To assess the combined effect of all blocks, we separately modeled the peak status outcome, first adding in those blocks with minimal impact in the individual additive models (blocks 1–3 and 5), then adding in block 4 (presenting vitals), separately adding in block 6 (inflammatory labs), and combining all blocks in the final model. We report the difference in the estimated sex effect (log odds ratio) with 95% bootstrapped confidence intervals of the difference. All analyses were performed using R, version 4.0.2 [31].
RESULTS
SARS-CoV-2 Testing and Demographics
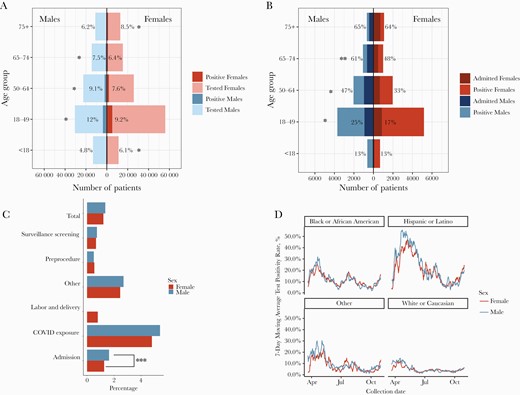
Between March and October 2020, there were 213 175 SARS-CoV-2 tests performed in the JHM system in individuals without a prior positive test result. Fifty-seven percent of tests were done in females and 43% in males, and the overall test positivity rate was 8% for females and 9% for males. Both test positivity and proportion of SARS-CoV-2-positive individuals requiring hospital admission were higher in males than females aged 18–74 (Figure 1A, B). Of the tests performed, 102 760 (48%) were done on asymptomatic (no COVID-19 symptoms) individuals (56% female vs 44% male). Asymptomatic test positivity overall was similar (1.4% male, 1.2% female; P = .05) (Figure 1C). The proportion of positive tests for males and females tracked closely together within race/ethnicity groups (Figure 1D).
A comparison of males and females stratified by age for (A) number of COVID-19 tests and proportion positive by age group and (B) proportion of those testing positive who required hospital admission. *P < .0001 and **P < .001, by chi-square; asterisk is on the side with the higher value. C, Test positivity rates by sex among individuals considered asymptomatic at the time of testing: ***P = .03, by chi-square. D, Seven-day moving average of test positivity rates for males and females in different race/ethnicity groups. Abbreviation: COVID-19, coronavirus disease 2019.
Clinical Presentation and Baseline Comorbidities Among Those Who Were Hospitalized
Detailed clinical and demographic data on the 2626 patients admitted with SARS-CoV-2 show a similar median age but an enrichment of females >75 years (Table 1). Admission from a nursing home, associated with severe outcomes in this cohort [27], was similar between males and females. Insurance status was distributed across multiple payor types, with more males in the “other” category (ie, worker’s compensation, Tricare, and other governmental programs). A greater proportion of females were Black, and a greater proportion of males were White. A higher proportion of males had severe disease (males 36% vs females 28%; P < .0001). Length of stay was ~0.5 days longer in males (P = .02), and time to severe or death outcome was ~5 hours longer in females (P = .04). There were numerically more females with DNR/DNI status within 24 hours of presentation.
Admission Cohort Characteristics Stratified by Sex
| . | Females . | Males . | P Value . |
|---|---|---|---|
| . | n = 1280 . | n = 1346 . | . |
| Median age (IQR),a y | 60 (42–76) | 59 (46–71) | .2 |
| Age distribution in strata, No. (%) | |||
| 18–49 y | 431 (34) | 413 (31) | .1 |
| 50–64 y | 300 (23) | 420 (31) | <.0001 |
| 65–74 y | 207 (16) | 269 (20) | .01 |
| >75 y | 342 (27) | 244 (18) | <.0001 |
| Admission from nursing home,b No. (%) | 193 (15) | 191 (14) | .6 |
| Insurance source,c No. (%) | .002 | ||
| Medicaid | 130 (15) | 120 (14) | .4 |
| Medicare | 368 (42) | 341 (38) | .09 |
| None | 49 (5.7) | 59 (6.7) | .4 |
| Other | 91 (11) | 151 (17) | <.001 |
| Private | 228 (26) | 216 (24) | .4 |
| Race/ethnicity,b No. (%) | .002 | ||
| Black | 504 (40) | 463 (34) | <.01 |
| White | 299 (23) | 400 (30) | <.001 |
| Hispanic | 128 (10) | 130 (9.7) | .8 |
| Other | 342 (27) | 350 (26) | .7 |
| Median BMI (IQR),a kg/m2 | 30 (25–36) | 28 (24–32) | <.001 |
| Charlson score, mean (SD)d | 1.8 (1.8) | 1.9 (1.8) | .7 |
| DNR/DNI within 24 h, No. (%) | 80 (6.2) | 61 (4.5) | .06 |
| Baseline medications with potential relevance to COVID-19 pathogenesis, No. (%) | |||
| ACE inhibitors | 114 (9.0) | 155 (12) | .03 |
| ARBs | 105 (8.3) | 100 (7.5) | .5 |
| Statins | 365 (29) | 425 (32) | .1 |
| Agents directed at COVID-19, No. (%) | |||
| Hydroxychloroquine | 187 (15) | 225 (17) | .2 |
| Remdesivir | 194 (15) | 235 (18) | .1 |
| Tocilizumab | 20 (1.6) | 74 (5.6) | <.0001 |
| Corticosteroids | 324 (26) | 317 (24) | .3 |
| Agents directed at coinfections, No. (%) | |||
| Antibiotics | 812 (64) | 884 (66) | .3 |
| Antifungals | 108 (8.5) | 97 (7.3) | .3 |
| Outcomes | |||
| Severe disease/death, No. (%) | 358 (28) | 482 (36) | <.0001 |
| Ventilated, No. (%) | 69 (5.5) | 107 (8.0) | .01 |
| Death, No. (%) | 149 (12) | 182 (14) | .2 |
| Median time to severe/death (IQR),a d | 0.84 (0.06–3.05) | 0.63 (0.02–2.76) | .04 |
| Median length of stay (IQR),a d | 5.5 (2.6–10.7) | 6.0 (2.9–11.6) | .02 |
| . | Females . | Males . | P Value . |
|---|---|---|---|
| . | n = 1280 . | n = 1346 . | . |
| Median age (IQR),a y | 60 (42–76) | 59 (46–71) | .2 |
| Age distribution in strata, No. (%) | |||
| 18–49 y | 431 (34) | 413 (31) | .1 |
| 50–64 y | 300 (23) | 420 (31) | <.0001 |
| 65–74 y | 207 (16) | 269 (20) | .01 |
| >75 y | 342 (27) | 244 (18) | <.0001 |
| Admission from nursing home,b No. (%) | 193 (15) | 191 (14) | .6 |
| Insurance source,c No. (%) | .002 | ||
| Medicaid | 130 (15) | 120 (14) | .4 |
| Medicare | 368 (42) | 341 (38) | .09 |
| None | 49 (5.7) | 59 (6.7) | .4 |
| Other | 91 (11) | 151 (17) | <.001 |
| Private | 228 (26) | 216 (24) | .4 |
| Race/ethnicity,b No. (%) | .002 | ||
| Black | 504 (40) | 463 (34) | <.01 |
| White | 299 (23) | 400 (30) | <.001 |
| Hispanic | 128 (10) | 130 (9.7) | .8 |
| Other | 342 (27) | 350 (26) | .7 |
| Median BMI (IQR),a kg/m2 | 30 (25–36) | 28 (24–32) | <.001 |
| Charlson score, mean (SD)d | 1.8 (1.8) | 1.9 (1.8) | .7 |
| DNR/DNI within 24 h, No. (%) | 80 (6.2) | 61 (4.5) | .06 |
| Baseline medications with potential relevance to COVID-19 pathogenesis, No. (%) | |||
| ACE inhibitors | 114 (9.0) | 155 (12) | .03 |
| ARBs | 105 (8.3) | 100 (7.5) | .5 |
| Statins | 365 (29) | 425 (32) | .1 |
| Agents directed at COVID-19, No. (%) | |||
| Hydroxychloroquine | 187 (15) | 225 (17) | .2 |
| Remdesivir | 194 (15) | 235 (18) | .1 |
| Tocilizumab | 20 (1.6) | 74 (5.6) | <.0001 |
| Corticosteroids | 324 (26) | 317 (24) | .3 |
| Agents directed at coinfections, No. (%) | |||
| Antibiotics | 812 (64) | 884 (66) | .3 |
| Antifungals | 108 (8.5) | 97 (7.3) | .3 |
| Outcomes | |||
| Severe disease/death, No. (%) | 358 (28) | 482 (36) | <.0001 |
| Ventilated, No. (%) | 69 (5.5) | 107 (8.0) | .01 |
| Death, No. (%) | 149 (12) | 182 (14) | .2 |
| Median time to severe/death (IQR),a d | 0.84 (0.06–3.05) | 0.63 (0.02–2.76) | .04 |
| Median length of stay (IQR),a d | 5.5 (2.6–10.7) | 6.0 (2.9–11.6) | .02 |
Statistics are chi-square, except as indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; DNR/DNI, do not resuscitate/do not intubate; IQR, interquartile range.
aCompared by Wilcoxon rank-sum test.
bAdmission source and race/ethnicity was available for >99% of cohort.
cInsurance source was not determined for ~30% of the cohort.
dCompared by t test.
Admission Cohort Characteristics Stratified by Sex
| . | Females . | Males . | P Value . |
|---|---|---|---|
| . | n = 1280 . | n = 1346 . | . |
| Median age (IQR),a y | 60 (42–76) | 59 (46–71) | .2 |
| Age distribution in strata, No. (%) | |||
| 18–49 y | 431 (34) | 413 (31) | .1 |
| 50–64 y | 300 (23) | 420 (31) | <.0001 |
| 65–74 y | 207 (16) | 269 (20) | .01 |
| >75 y | 342 (27) | 244 (18) | <.0001 |
| Admission from nursing home,b No. (%) | 193 (15) | 191 (14) | .6 |
| Insurance source,c No. (%) | .002 | ||
| Medicaid | 130 (15) | 120 (14) | .4 |
| Medicare | 368 (42) | 341 (38) | .09 |
| None | 49 (5.7) | 59 (6.7) | .4 |
| Other | 91 (11) | 151 (17) | <.001 |
| Private | 228 (26) | 216 (24) | .4 |
| Race/ethnicity,b No. (%) | .002 | ||
| Black | 504 (40) | 463 (34) | <.01 |
| White | 299 (23) | 400 (30) | <.001 |
| Hispanic | 128 (10) | 130 (9.7) | .8 |
| Other | 342 (27) | 350 (26) | .7 |
| Median BMI (IQR),a kg/m2 | 30 (25–36) | 28 (24–32) | <.001 |
| Charlson score, mean (SD)d | 1.8 (1.8) | 1.9 (1.8) | .7 |
| DNR/DNI within 24 h, No. (%) | 80 (6.2) | 61 (4.5) | .06 |
| Baseline medications with potential relevance to COVID-19 pathogenesis, No. (%) | |||
| ACE inhibitors | 114 (9.0) | 155 (12) | .03 |
| ARBs | 105 (8.3) | 100 (7.5) | .5 |
| Statins | 365 (29) | 425 (32) | .1 |
| Agents directed at COVID-19, No. (%) | |||
| Hydroxychloroquine | 187 (15) | 225 (17) | .2 |
| Remdesivir | 194 (15) | 235 (18) | .1 |
| Tocilizumab | 20 (1.6) | 74 (5.6) | <.0001 |
| Corticosteroids | 324 (26) | 317 (24) | .3 |
| Agents directed at coinfections, No. (%) | |||
| Antibiotics | 812 (64) | 884 (66) | .3 |
| Antifungals | 108 (8.5) | 97 (7.3) | .3 |
| Outcomes | |||
| Severe disease/death, No. (%) | 358 (28) | 482 (36) | <.0001 |
| Ventilated, No. (%) | 69 (5.5) | 107 (8.0) | .01 |
| Death, No. (%) | 149 (12) | 182 (14) | .2 |
| Median time to severe/death (IQR),a d | 0.84 (0.06–3.05) | 0.63 (0.02–2.76) | .04 |
| Median length of stay (IQR),a d | 5.5 (2.6–10.7) | 6.0 (2.9–11.6) | .02 |
| . | Females . | Males . | P Value . |
|---|---|---|---|
| . | n = 1280 . | n = 1346 . | . |
| Median age (IQR),a y | 60 (42–76) | 59 (46–71) | .2 |
| Age distribution in strata, No. (%) | |||
| 18–49 y | 431 (34) | 413 (31) | .1 |
| 50–64 y | 300 (23) | 420 (31) | <.0001 |
| 65–74 y | 207 (16) | 269 (20) | .01 |
| >75 y | 342 (27) | 244 (18) | <.0001 |
| Admission from nursing home,b No. (%) | 193 (15) | 191 (14) | .6 |
| Insurance source,c No. (%) | .002 | ||
| Medicaid | 130 (15) | 120 (14) | .4 |
| Medicare | 368 (42) | 341 (38) | .09 |
| None | 49 (5.7) | 59 (6.7) | .4 |
| Other | 91 (11) | 151 (17) | <.001 |
| Private | 228 (26) | 216 (24) | .4 |
| Race/ethnicity,b No. (%) | .002 | ||
| Black | 504 (40) | 463 (34) | <.01 |
| White | 299 (23) | 400 (30) | <.001 |
| Hispanic | 128 (10) | 130 (9.7) | .8 |
| Other | 342 (27) | 350 (26) | .7 |
| Median BMI (IQR),a kg/m2 | 30 (25–36) | 28 (24–32) | <.001 |
| Charlson score, mean (SD)d | 1.8 (1.8) | 1.9 (1.8) | .7 |
| DNR/DNI within 24 h, No. (%) | 80 (6.2) | 61 (4.5) | .06 |
| Baseline medications with potential relevance to COVID-19 pathogenesis, No. (%) | |||
| ACE inhibitors | 114 (9.0) | 155 (12) | .03 |
| ARBs | 105 (8.3) | 100 (7.5) | .5 |
| Statins | 365 (29) | 425 (32) | .1 |
| Agents directed at COVID-19, No. (%) | |||
| Hydroxychloroquine | 187 (15) | 225 (17) | .2 |
| Remdesivir | 194 (15) | 235 (18) | .1 |
| Tocilizumab | 20 (1.6) | 74 (5.6) | <.0001 |
| Corticosteroids | 324 (26) | 317 (24) | .3 |
| Agents directed at coinfections, No. (%) | |||
| Antibiotics | 812 (64) | 884 (66) | .3 |
| Antifungals | 108 (8.5) | 97 (7.3) | .3 |
| Outcomes | |||
| Severe disease/death, No. (%) | 358 (28) | 482 (36) | <.0001 |
| Ventilated, No. (%) | 69 (5.5) | 107 (8.0) | .01 |
| Death, No. (%) | 149 (12) | 182 (14) | .2 |
| Median time to severe/death (IQR),a d | 0.84 (0.06–3.05) | 0.63 (0.02–2.76) | .04 |
| Median length of stay (IQR),a d | 5.5 (2.6–10.7) | 6.0 (2.9–11.6) | .02 |
Statistics are chi-square, except as indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; DNR/DNI, do not resuscitate/do not intubate; IQR, interquartile range.
aCompared by Wilcoxon rank-sum test.
bAdmission source and race/ethnicity was available for >99% of cohort.
cInsurance source was not determined for ~30% of the cohort.
dCompared by t test.
Overall, symptoms reported on presentation were similar, but a greater proportion of males reported fevers (P < .05), whereas females had a greater frequency of headache (P < .001), loss of smell (P < .05), and vomiting (P < .001) (Supplementary Figure 1). At presentation, more males had a temperature >38.0°C (P < .001), and females had more favorable respiratory parameters: lower respiratory rates (RRs), lower levels of supplemental O2, and a greater SpO2:FiO2 ratio across all age groups (384 vs 364; P < .001). In the subset of individuals who developed severe disease/death (n = 843, 32%), the sex differences in presenting vital signs were attenuated (Supplementary Table 1). At both 6 and 24 hours after admission, females were more frequently classified as mild/moderate than males (Supplementary Table 2).
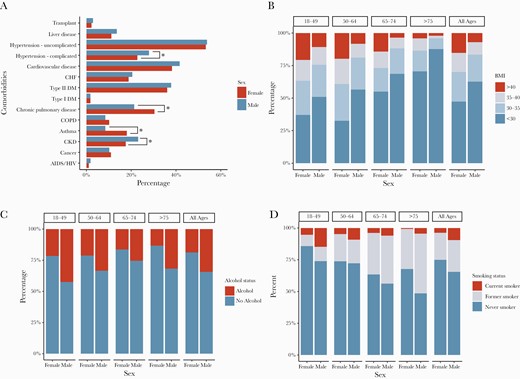
The overall burden of comorbid conditions (Charlson score) was similar between males and females (Table 1). However, significant differences in specific comorbidities were observed: Chronic lung disease and asthma were more prevalent among females (P < .001), females had higher BMI, and males more frequently had chronic kidney disease and complicated hypertension. Males also had a higher frequency of both smoking and alcohol use (Figure 2; Supplemental Table 3).
Frequency of comorbid conditions at baseline for males and females, with several comorbidities presenting with a sex imbalance. A, *P < .05 and **P < .001, by chi-square. Distribution of BMI categories (B), alcohol use (C), and smoking status (D) by sex and age. Overall chi-square for (B), (C), and (D): P < .001. Abbreviations: BMI, body mass index; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus.
Laboratory Measures
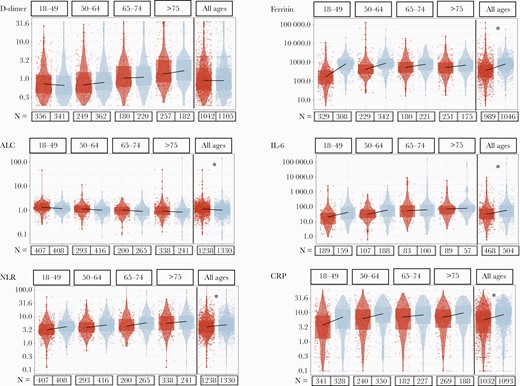
At presentation, females and males had comparable levels of D-dimer and erythrocyte sedimentation rate (ESR), but males had lower absolute lymphocyte counts, higher neutrophil:lymphocyte ratios (NLRs), and higher ferritin, IL-6, and CRP levels than females (P < .001, Wilcoxon rank-sum test) (Figure 3). Age-stratified analyses demonstrated differential effects of age; with increasing age, there was less difference between the median values of males and females for CRP, ferritin, and IL-6 (Figure 3). To assess whether this difference was related to timing in disease course and time of presentation, we assessed the peak (ie, CRP, ferritin, IL-6, NLR) or nadir (ie, ALC) levels over the course of admission and observed the same patterns of lower markers of inflammation in females (Supplementary Table 4). We then analyzed only the subset of individuals who reached severe disease or death during their hospitalization and again observed greater levels of inflammatory responses at presentation for the same markers and similar trends across the age strata (Supplementary Table 5, Supplementary Figure 2).
Median lab values at the time of admission (–48 hours to +48 hours) by sex and age. Red represents females, and blue represents males; total numbers of observations for each lab are indicated below the column, and the right-most column is the overall sex comparison. Statistics on the overall comparison: *P < .001, by Wilcoxon rank test. Abbreviations: ALC, absolute lymphocyte count; CRP, C-reactive protein; IL-6, interleukin-6; NLR, neutrophil:lymphocyte ratio.
Medication Use
We identified use of medications postulated to be relevant to SARS-CoV-2 infection. Baseline use of statins and ARBs was comparable between sexes, but males had higher ACE inhibitor use (12% males vs 9% females; P = .03) (Table1). Analysis of medications intended as direct therapeutics for COVID-19 revealed similar usage of remdesivir, hydroxychloroquine, and steroids in males and females and more use of tocilizumab in males (P < .0001) in the small group of individuals (n = 94) who received this therapy (Table1).
Modeling the Impact of Sex on the Risk of Severe Disease
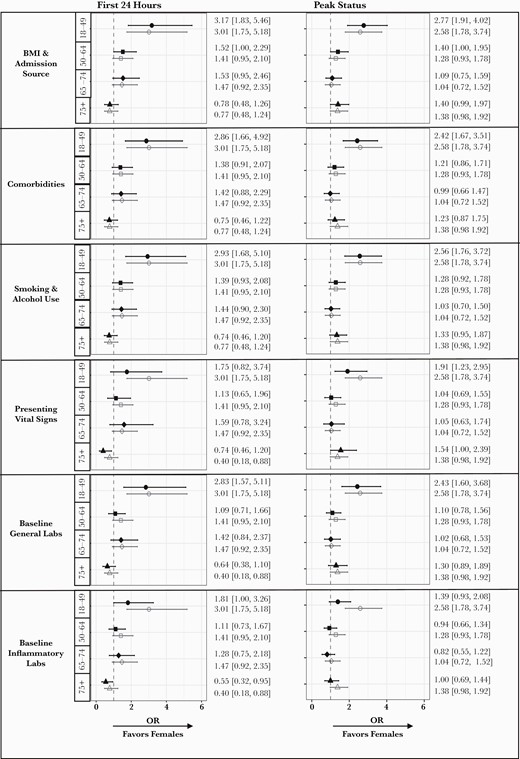
To assess the impact of sex on the risk for severe COVID-19 or death, we developed an age-stratified baseline logistic regression model including race/ethnicity and admitting hospital. We tested the early impact of sex (ie, within 24 hours of presentation) and found that the OR (95% CI) for severe disease/death at 24 hours for males relative to females was 3.01 (1.75 to 5.18) in those age 18–49 years, 1.41 (0.95 to 2.10) in those age 50–64 years, 1.47 (0.92 to 2.35) in those age 65–74 years, and 0.77 (0.48 to 1.24) in those age >75 years (Figure 4). We then used a block approach to test the impact of other variables on the sex effect. Each block addition (black-filled symbol) is shown in Figure 4 in reference to the baseline model (gray open symbols). With the addition of blocks for BMI/admission source, comorbidities, and health behaviors (ie, smoking and alcohol use) and baseline general health status labs, there was minimal change in the OR of severe COVID-19 outcomes. Adding the presenting vital signs shifted the estimated sex effect, consistent with the known predictive value of respiratory parameters and hypoxemia in severe outcomes. The addition of the block of inflammatory laboratory values substantially reduced the OR of risk of severe outcomes for males, most prominently in the 18–49 age group (Figure 4). To quantify the impact of block addition, we assessed the change in log OR with confidence intervals for each block addition. Across all the ages, addition of the inflammatory labs was the only block that significantly changed the estimated sex effect (P < .0001) (Supplementary Figure 3).
Odds ratio of increased risk for severe or death outcomes in males stratified by age. The risk of male sex is represented for the baseline model (open gray symbol in each graph) and then after addition of each block of variables (black-filled symbol) for a model of outcomes at 24 hours and at peak status during hospitalization. The numbers indicate the point estimate for the OR and the 95% CI. Abbreviations: BMI, body mass index; OR, odds ratio.
The same analysis was repeated to assess sex differences in peak disease status at any point during hospitalization. The OR (95% CI) of severe/death outcomes was significantly elevated in males aged 18–49 years (2.58; 1.78 to 3.74) (Figure 4). Again, the initial inflammatory labs substantially shifted the adjusted OR for severe outcomes associated with male sex in particular in the 18–49-year-old age stratum (1.39; 0.93 to 2.08). Across all ages, the addition of the inflammatory lab block was again the only block associated with a statistically significant change in the log odds (P < 1e10-14) (Supplementary Figure 3). In both models, controlling for vitals at presentation did not have a statistically significant impact on the sex effect, and the increased risk of severe COVID-19 outcomes in males was primarily in the 18–49-year age stratum. Excluding pregnant women or individuals with DNR/DNI status within 24 hours did not substantially change the analytic findings (data not shown).
To assess the collective impact of all variables, we focused on the 18–49-year age group and built a combined model for the peak status outcome. We estimated the difference in the sex effect between the base model and multiple combinations of the variable blocks and obtained a confidence interval for the difference across models using bootstrapping analysis. We first added blocks 1–3 and 5 (BMI/age, comorbidities, smoking status/alcohol (EtOH) use, baseline general labs), which individually had a minimal impact on the sex effect. In the combined model this held true, with a change in log odds (95% CI) of 0.05 (–0.17 to 0.27) over the base model. Adding in presenting vital signs (blocks 1–5) led to a change in log odds (95% CI) of 0.22 (–0.15 to 0.59); separately adding the inflammatory labs (blocks 1–3, 5–6) led to the most substantial impact on the sex effect, with a change in log odds (95% CI) of 0.47 (0.21 to 0.73) over the base model. The combined model of all blocks (1–6) yielded a change in log odds (95% CI) of 0.38 (–0.01 to 0.76). Consistent with the individual additive models, the combined model identifies the inflammatory labs as having the most substantial impact on the estimated sex effect even when combined with other variable blocks (Supplementary Figure 4).
DISCUSSION
In this study, we leverage the power of a large hospital system with detailed patient-level data to elucidate features of COVID-19 infection that differ between males and females from diagnosis to outcomes. Our data are consistent with prior reports of more severe disease in males [3, 32–36], but our analysis adds novel insights into asymptomatic test positivity rates and differential features of hospital presentation and identifies inflammatory markers as the most significant modifier of the differential risk of severe COVID-19 outcomes. Our data suggest that there is a fundamental difference in the immune inflammatory response to SARS-CoV-2 infection that is advantageous in females, in particular in females of reproductive age.
Consistent with other studies, we show higher rates of both hospitalization and severe outcomes [3, 34, 36, 37]. An unresolved question is whether the difference extends to the level of asymptomatic infection; in our data it did not, as the rate of positivity was comparable between males and females. This result is discordant from a serosurvey of individuals without a known history of SARS-CoV-2 or recalled symptoms, which found higher seropositivity among females [38]. In our study, asymptomatic cases were identified during infection with direct SARS-CoV-2 RNA testing and would be sensitive to a lower viral load or shorter window of viral shedding. While our data set has the advantage of large numbers of asymptomatic tests (>100 000), we cannot account for gender-based differences in seeking testing or other health care. Further work is needed to define whether there are differences in asymptomatic infection and to reconcile different modes of detecting infection.
Our detailed clinical data allowed us to assess sex differences in severity at presentation and treatment interventions. Males had more severe illness at presentation, but the minimal difference in time to peak disease status (~5 hours) argues against a gender-based difference in the timing of seeking care. Likewise, the difference in inflammatory labs both at presentation and peak/nadir suggests that the sex difference is not only timing but also in the severity of the illness. Most therapeutics were used with similar frequency in males and females, suggesting that this did not drive a difference in outcomes. Although we observed more frequent use of tocilizumab in males, this group size was small. Multiple factors converge on the decision to use an investigational therapeutic, including clinician and patient decision-making and the biomedical features of disease, and we cannot draw definitive conclusions about the role of sex/gender in this observation. However, there is a paucity of research into the sex-specific use or efficacy of therapies directed at COVID-19 [39] despite the observation that subgroup analyses of both dexamethasone [40] and tocilizumab [41] in the RECOVERY trial did not show a significant benefit for females from therapy. Sex-specific analysis of the use of therapeutics and careful analysis of clinical trial data may determine whether there are sex-specific inflammatory thresholds that could guide use of anti-inflammatory agents.
In prior studies identifying an increased risk for severe outcomes, burden or type of comorbidities or other health behaviors has been suggested as a mechanism for the sex effect. In our data, a number of comorbidities were unbalanced, notably obesity, which has been independently associated with severe outcomes [27, 35]. To our surprise, the addition of these comorbidities and health behaviors had no impact on the increased risk for severe disease in males in our models. This is consistent with a prior multinational study in which male mortality was higher even with propensity score matching [42] and extended by the additional variables our study assessed. Our analytic approach of adding variables by block allowed us to broadly query potential sources of variation and uniquely identify the sex differential inflammatory labs as the main feature impacting the male sex effect on severe disease, and the focusing of that risk in individuals of reproductive age (18–49). In our combined model, focusing on the 18–49-year-old age stratum, again the inflammatory lab values had the most substantial impact on the sex effect.
Limitations
These data are consistent with a differential inflammatory response to SARS-CoV-2 infection as a contributor to increased male risk; however, this is an observational cohort, and further studies would be necessary to establish a causal role. In addition, the inflammatory indices used in these analyses were ordered based on clinician discretion and were not available for all individuals in the cohort. While these data suggest a specific effect in the reproductive age group, we did not have direct data on menopausal status or exogenous hormone exposure; our data do not allow us to conclude whether hormones are directly mechanistic in outcome differences. Further work is also needed to explore the role of sex in the oldest age stratum (age >75) where differences in DNR/DNI status and longevity may have an impact on outcomes. Our data represent the specific population and epidemic in the Baltimore–Washington DC area and may not be generalizable.
CONCLUSIONS
Our analysis confirms an excess of hospital admission and severe/death outcomes from COVID-19 among males, in particular in the 18–49 age group. With detailed patient-level data, we find that the sex effect is most strongly linked to inflammatory profile and not to expected sociodemographic and other clinical characteristics. Our data suggest that differences in the immune response to the virus should be a primary focus of future mechanistic studies to identify whether sex steroid hormones, gene expression differences, or a combination of these factors drives the overall female advantage in COVID-19 [6].
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the efforts of many people throughout Johns Hopkins who have worked to focus resources on the response to the COVID-19 epidemic during this past year.
Financial support. The data utilized were part of JH-CROWN: The COVID PMAP Registry, which is based on the contribution of many patients and clinicians and is funded by Hopkins inHealth, the Johns Hopkins Precision Medicine Program. Drs. Garibaldi, Muschelli, Robinson, Bandeen-Roche, and Gupta and Mr. Schumock received funding from the COVID-19 Administrative Supplement for the Health and Human Services Region 3 Treatment Center from the Office of the Assistant Secretary for Preparedness and Response. Drs. Klein and Zeger and Mr. Betz received funding from the National Institute of Health/National Cancer Institute–funded COVID-19 Serology Center of Excellence (U54CA260492). Dr. Scully is supported by R01AI154541 (National Institute of Allergy and Infectious Diseases/Office for Research on Women’s Health). Dr. Gupta was also supported by National Institute of Allergy and Infectious Diseases UM1AI069645. The funders had no role in the design, analysis, or conduct of the study or in the decision to submit the manuscript for publication.
Potential conflicts of interest. The authors have no relevant conflicts to disclose. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. E.P.S., S.L.K., and A.G. conceived of the study, and together with G.S., M.F., G.M., K.B.R., and S.Z. designed the statistical analysis plan. G.S., M.F., G.M., J.M., J.B., and N.W. contributed to data extraction, cleaning, analysis, diagnosis verification and presentation. E.K. contributed testing and admission data. B.T.G., M.R., J.M., and J.B. contributed to the creation and maintenance of the JH-CROWN Registry. All authors contributed to the writing and editing of the manuscript. E.P.S., G.S., and M.F. had full access to all the data and are responsible for the integrity of the data and accuracy of analysis.
References
Substance Abuse and Mental Health Services Administration.
Author notes
Equal contribution





Comments