-
PDF
- Split View
-
Views
-
Cite
Cite
Kostas Danis, Olivier Epaulard, Thomas Bénet, Alexandre Gaymard, Séphora Campoy, Elisabeth Botelho-Nevers, Maude Bouscambert-Duchamp, Guillaume Spaccaferri, Florence Ader, Alexandra Mailles, Zoubida Boudalaa, Violaine Tolsma, Julien Berra, Sophie Vaux, Emmanuel Forestier, Caroline Landelle, Erica Fougere, Alexandra Thabuis, Philippe Berthelot, Raphael Veil, Daniel Levy-Bruhl, Christian Chidiac, Bruno Lina, Bruno Coignard, Christine Saura, Investigation Team , Cluster of Coronavirus Disease 2019 (COVID-19) in the French Alps, February 2020, Clinical Infectious Diseases, Volume 71, Issue 15, 1 August 2020, Pages 825–832, https://doi.org/10.1093/cid/ciaa424
Close - Share Icon Share
Abstract
On 7 February 2020, French Health authorities were informed of a confirmed case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in an Englishman infected in Singapore who had recently stayed in a chalet in the French Alps. We conducted an investigation to identify secondary cases and interrupt transmission.
We defined as a confirmed case a person linked to the chalet with a positive reverse-transcription polymerase chain reaction sample for SARS-CoV-2.
The index case stayed 4 days in the chalet with 10 English tourists and a family of 5 French residents; SARS-CoV-2 was detected in 5 individuals in France, 6 in England (including the index case), and 1 in Spain (overall attack rate in the chalet: 75%). One pediatric case, with picornavirus and influenza A coinfection, visited 3 different schools while symptomatic. One case was asymptomatic, with similar viral load as that of a symptomatic case. Seven days after the first cases were diagnosed, 1 tertiary case was detected in a symptomatic patient with from the chalet a positive endotracheal aspirate; all previous and concurrent nasopharyngeal specimens were negative. Additionally, 172 contacts were monitored; all contacts tested for SARS-CoV-2 (N = 73) were negative.
The occurrence in this cluster of 1 asymptomatic case with similar viral load as a symptomatic patient suggests transmission potential of asymptomatic individuals. The fact that an infected child did not transmit the disease despite close interactions within schools suggests potential different transmission dynamics in children. Finally, the dissociation between upper and lower respiratory tract results underscores the need for close monitoring of the clinical evolution of suspected cases of coronavirus disease 2019.
A novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) causing respiratory infections (coronavirus disease 2019 [COVID-19]) was first detected in Wuhan, China in December 2019 [1–3]. Within weeks, the virus spread to all provinces of China, and to a growing number of countries in 5 continents (China accounting for >99% of cases) [4, 5]. The first reported case series featured a high proportion of severe disease (acute respiratory distress syndrome in 17%–29% of the cases) [6, 7]. On 24 January 2020, the first 3 confirmed COVID-19 cases were detected in France, being the first reported in Europe [8, 9]; as of 7 February 2020, a total of 6 cases had been detected: 4 among individuals coming or returning from China and 2 among nontravelers in contact with cases in France.
On 7 February 2020, the French health authorities were informed via the European Early Warning and Response System (EWRS) of a confirmed COVID-19 case in the United Kingdom (UK) on 6 February 2020, and who had recently stayed in France. During 18–23 January 2020, the index case had attended a conference in Singapore linked to COVID-19 confirmed cases, the first of whom was from Wuhan, China. On 24 January 2020, he flew from London to Geneva for a skiing holiday in France. He stayed for 1 night in Saint-Gervais-les-Bains, Haute-Savoie, and then in a chalet in the ski resort of Contamines-Montjoie, Haute-Savoie. On 28 January 2020, he flew back to the UK from Geneva. He developed moderate symptoms during the night of 24 January 2020 and was symptomatic during the rest of his stay in France. On 7 February 2020, we initiated an investigation to detect potential secondary COVID-19 cases, identify their contacts, and prevent transmission.
METHODS
Case Definitions
In accordance with the 7 February 2020 surveillance case definition [10], we defined as a confirmed case a person with a positive SARS-CoV-2 reverse-transcription polymerase chain reaction (RT-PCR) result on respiratory samples and a direct or indirect epidemiological link with the chalet in Contamines-Montjoie. A possible case was a patient with an acute respiratory illness, whatever the severity, and a direct or indirect epidemiological link with a confirmed case from the chalet.
Contact Tracing and Data Collection
Confirmed cases were interviewed using bespoke questionnaires on exposure and clinical history, clinical characteristics, and their contacts during the period of their clinical symptoms. Depending on the level of risk, we defined contacts as high/moderate, low, or negligible risk, as previously reported [8]. As per the French national guidance for contacts of COVID-19 cases [8], low-risk contacts were asked to measure their body temperature twice a day during a 14-day period after their last exposure and, in case of fever or respiratory symptoms, to wear a surgical mask and contact the emergency hotline (Medical emergency service center 15). In addition, high/moderate-risk contacts were isolated at home, and were actively followed up through daily calls.
Laboratory Methods
Respiratory samples were either nasopharyngeal (NP) swabs (upper respiratory tract) (Sigma Virocult, Medical Wire Instrument, UK) or endotracheal aspirates (lower respiratory tract). All samples were refrigerated and shipped to the National Reference Center for Respiratory Viruses (Hospices Civils de Lyon), where they were tested for SARS-CoV-2 and other respiratory pathogens [11]. Details of the procedures of RNA extraction and real-time RT-PCR and of the viral load calculation are presented in the Supplementary Materials.
Ethical Considerations
Investigations complied with the General Data Protection Regulation (Regulation [European Union] 2016/679 and Directive 95/46/EC) and the French data protection law (law number 78-17 on 6 January 1978 and Décret number 2019-536 on 29 May 2019). Informed consent to disclose information relevant to this publication was obtained from the confirmed cases in France.
RESULTS
Cases and Contacts in the Chalet (Les Contamines-Montjoie)
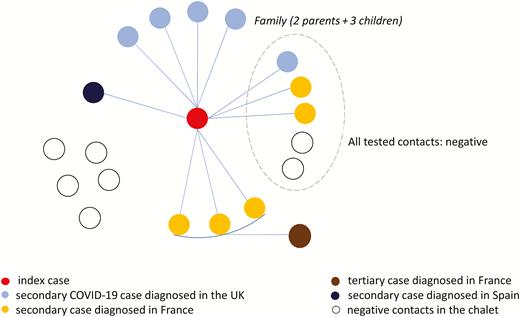
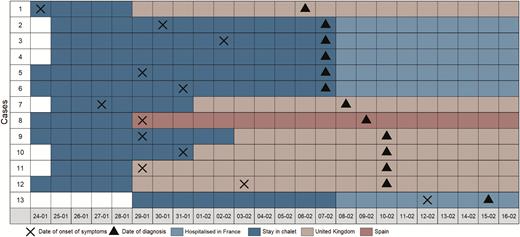
During 25–28 January 2020, the index case stayed in the chalet with 10 other adult British tourists (apartment 1) and a British family of 2 adults and 3 children, residents in France (apartment 2) (Figure 1). Of these, 5 tested positive for SARS-CoV-2 in France, 6 were found positive in the UK (including the index case), and 1 in Spain, indicating an overall attack rate of 75% (12/16) among the tourists/residents in those apartments, 82% (9/11) in apartment 1, and 60% (3/5) in apartment 2) (Figures 1 and 2). In addition, 5 other adult British tourists arrived in apartment 1 of the chalet after the index case had left (high-risk contacts of the secondary cases). On 7 February 2020, 4 of those adult British tourists in apartment 1 and the 2 children of the family in apartment 2 were isolated in hospitals; 1 adult tourist had left for the UK before 7 February and was followed up there. On 15 February, 1 of those adult tourists tested positive while being in hospital isolation in France; he had stayed in apartment 1 of the chalet together with 3 secondary cases (tertiary case). All cases were adults, apart from a 9-year-old child who attended 3 different schools (schools A, B, and C) and 1 ski class while symptomatic. Of all cases, 75% were males. The dates of onset of symptoms of the confirmed cases, excluding the index case, ranged from 27 January to 12 February (Figure 2).
Cluster of coronavirus disease 2019, Contamines-Montjoie, France, January–February 2020. Abbreviations: COVID-19, coronavirus disease 2019; UK, United Kingdom.
Temporal relation between coronavirus disease 2019 cases in the chalet and onset of symptoms of cases, Contamines-Montjoie, France, January–February 2020. Cases 2–6 were first tested on 7 February 2020 and were diagnosed positive on the same day.
Clinical Characteristics and Management of Cases Diagnosed in France (n = 6)
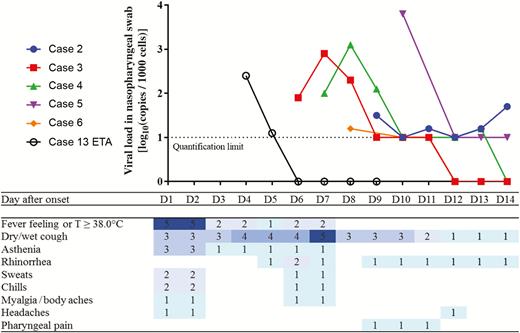
Of all 6 confirmed cases diagnosed in France, case 4 did not develop any sign or symptom, apart from muscle pain on 27 January 2020 following intense sport activities. Among the other 5 symptomatic confirmed cases, the following signs and symptoms were reported: fever (n = 5), dry cough (n = 5), wet cough (n = 1), asthenia/fatigue (n = 3), chills (n = 2), sweats (n = 2), rhinorrhea (n = 3), and myalgia (n = 1); 4 cases had those mild signs/symptoms 1 week before the first positive test on 7 February (Figures 3 and 4). At the time of diagnosis, they had these symptoms with an even lower intensity, and all had a benign clinical evolution. Case 13, a high-risk contact initially negative for SARS-CoV-2, developed fever and cough with respiratory crackles at auscultation on the fifth day of hospital isolation. A bilateral interstitial syndrome was identified at the computed tomography (CT) scan with bilateral ground glass opacification predominating on the left. The symptoms of all cases resolved rapidly, without antiviral treatment.
Normalized viral loads and symptoms of confirmed coronavirus disease 2019 (COVID-19) cases by day since onset of symptoms, Contamines-Montjoie, France, January–February 2020. Cases 2–6: All virological data were obtained from nasopharyngeal swabs. Case 4 was asymptomatic; for visual purposes, we arbitrarily assigned 31 January as day 1 (D1), as it was the median of the day of onset of the other (symptomatic) cases. Case 13: Virological data were obtained from endotracheal aspirates (ETAs), all nasopharyngeal swabs being negative (before or during the period of positive ETAs). Results below the quantification limit are positive but nonquantifiable. D1 corresponds to the date of onset of COVID-19 symptoms.
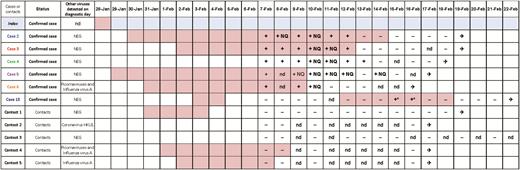
Duration of symptoms and virological results for the 6 confirmed coronavirus disease 2019 cases in France and their contacts in the chalet in Contamines-Montjoie, January–February 2020. Symptomatic period for each confirmed case and contact/patient in the United Kingdom. All virological data were obtained from nasopharyngeal swabs, unless specified. The asterisk (*) indicates results on endotracheal aspirates. On 7 February, all cases and contacts were tested for other respiratory viruses; on 13 February, case 13 was retested for other respiratory viruses. All cases and contacts from the 2 apartments were discharged from hospital. Abbreviations: ✈, hospital discharge; −, negative; +, positive and quantified; +NQ, positive but not quantifiable; nd, not determined; NEG, negative.
Virological Findings of Cases
All 5 symptomatic confirmed cases were first tested between 6 and 10 days after symptom onset. For 4 cases, a low viral load detected (often under the limit of quantification of 1 log10 copies/1000 cells) (Figures 3 and 4). All confirmed cases stopped viral excretion within 17 days after onset of illness (median, 11 days [range, 5–17 days]) (Figure 4). Case 4, the asymptomatic case, had the same natural history of viral excretion as symptomatic case 3 with a viral shedding of 7 and 6 days, respectively. Cases 2 and 6 (child) had similar shedding patterns, with viral load close to the limit of quantification for several days before being negative, much lower than that observed in cases 3 and 4. Case 13 had an endotracheal aspirate (ETA) positive at D4 with SARS-CoV-2 (viral load of 2.4 log10 copies/1000 cells), while negative in the NP swabs collected since admission (8 days). The NP swabs of the same day and the following days remained negative. The daily follow-up revealed a short-lasting excretion with only 2 successive ETAs with a lower viral load (2.4 log10 and 1.1 log10 copies/1000 cells, respectively; Figure 4), and the subsequent ETA remained negative. All cases were negative for other viruses except for the pediatric case, who had a SARS-CoV-2 + picornavirus (rhinovirus or enterovirus) + influenza A(H1N1)pdm09 coinfection. His 2 siblings were negative for SARS-CoV-2, but had an influenza A(H1N1)pdm09 infection and an influenza A(H1N1)pdm09 + picornavirus coinfection, respectively.
Control Measures and Contact-Tracing Activities in France
Because of the large number of contacts of the pediatric case (case 6), particular attention was paid to detect tertiary cases in children in the 3 schools the child attended while symptomatic. On 8 February, a public meeting was held to inform the parents of 2 schools (A and B); the parents of school C were informed by telephone. As a precaution, the first 2 schools were closed for 2 weeks and the third for 1 week (end of follow-up period; the pediatric case visited that school on 31 January). On Sunday 9 February, infectious disease specialists and epidemiologists evaluated the risk of 112 school contacts. All children and teachers who were in the same class as the symptomatic pediatric case were considered as high-risk contacts and were isolated at home (Table 1). All hospitals in the region implemented emergency plans to accommodate potential tertiary cases.
Overall, 172 contacts were identified; 84 (49%) were classified as high/moderate risk and 88 (51%) as low risk (Table 1). Of those, 98% (n = 169) were contacted; 70 (41%) had respiratory symptoms during the investigation and were thus classified as possible cases; 73 were tested; all tested negative for SARS-CoV-2 except for case 13, who tested positive during hospitalization. No additional cases were identified within the 14-day follow-up period of all the contacts. The movement history of the confirmed cases during their stay in France and their contact tracing is presented in the Supplementary Materials. In brief, contacts of the other 5 cases that were monitored included teachers in another school, apartment staff and cleaners, staff in shops and restaurants, and passengers in 4 buses and 3 airplanes.
Number of High-risk and Low-risk Contacts and Virological Results for Respiratory Viruses, Contamines-Montjoie, France, January–February 2020
| Risk Category . | Contacts, No. . | Contacteda . | Possible Casesa . | Sampleda . | SARS-CoV-2b . | Adenovirusb . | Human Coronavirusb . | Influenzab . | Parainfluenzab . | Picornavirusb . |
|---|---|---|---|---|---|---|---|---|---|---|
| School A | ||||||||||
| High risk | 31 | 31 (100) | 18 (58) | 19 (61%)c | 0 (0) | 0 (0) | 6 (32): 2 HKU1, 4 NL63 | 6 (32): 3 IAV, 3 IBV | 3 (16): 3 PIV2 | 3 (16) |
| Low risk | 24 | 24 (100) | 23 (96) | 23 (96) | 0 (0) | 1 (4) | 2 (9): 2 HKU1 | 11 (48): 10 IAV, 1 IBV | 1 (4): 1 PIV2 | 3 (13) |
| School B | ||||||||||
| High risk | 5 | 5 (100) | 2 (40) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| School C | ||||||||||
| High risk | 25 | 25 (100) | 10 (40) | 10 (40) | 0 (0) | 0 (0) | 0 (0) | 3 (30): 1 IAV, 2 IBV | 0 (0) | 3 (30) |
| Low risk | 1 | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Ski club | ||||||||||
| High risk | 5 | 5 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Low risk | 11 | 10 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Other community contacts | ||||||||||
| High risk | 12 | 11 (92) | 8 (67) | 8 (67) | 0 (0) | 1 (13) | 2 (25): 1 HKU1, 1 NL63 | 2 (25): 2 IAV | 0 (0) | 1 (13) |
| Low risk | 52 | 51 (98) | 4 (8) | 4 (8) | 0 (0) | 0 (0) | 1 (25): 1 NL63 | 0 (0) | 2 (50): 1 PIV2 | 1 (25) |
| Hospitalized contactsd | ||||||||||
| High risk | 6 | 6 (100) | 4 (67) | 6 (100) | 1 (17) | 0 (0) | 1 (17): 1 HKU1 | 2 (33): 2 IAV | 0 (0) | 1 (17) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| Total | 172 | 168 (98) | 70 (40) | 73 (42)c | 1 (1) | 2 (3) | 12 (16) | 24 (33) | 6 (8) | 13 (18) |
| Risk Category . | Contacts, No. . | Contacteda . | Possible Casesa . | Sampleda . | SARS-CoV-2b . | Adenovirusb . | Human Coronavirusb . | Influenzab . | Parainfluenzab . | Picornavirusb . |
|---|---|---|---|---|---|---|---|---|---|---|
| School A | ||||||||||
| High risk | 31 | 31 (100) | 18 (58) | 19 (61%)c | 0 (0) | 0 (0) | 6 (32): 2 HKU1, 4 NL63 | 6 (32): 3 IAV, 3 IBV | 3 (16): 3 PIV2 | 3 (16) |
| Low risk | 24 | 24 (100) | 23 (96) | 23 (96) | 0 (0) | 1 (4) | 2 (9): 2 HKU1 | 11 (48): 10 IAV, 1 IBV | 1 (4): 1 PIV2 | 3 (13) |
| School B | ||||||||||
| High risk | 5 | 5 (100) | 2 (40) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| School C | ||||||||||
| High risk | 25 | 25 (100) | 10 (40) | 10 (40) | 0 (0) | 0 (0) | 0 (0) | 3 (30): 1 IAV, 2 IBV | 0 (0) | 3 (30) |
| Low risk | 1 | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Ski club | ||||||||||
| High risk | 5 | 5 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Low risk | 11 | 10 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Other community contacts | ||||||||||
| High risk | 12 | 11 (92) | 8 (67) | 8 (67) | 0 (0) | 1 (13) | 2 (25): 1 HKU1, 1 NL63 | 2 (25): 2 IAV | 0 (0) | 1 (13) |
| Low risk | 52 | 51 (98) | 4 (8) | 4 (8) | 0 (0) | 0 (0) | 1 (25): 1 NL63 | 0 (0) | 2 (50): 1 PIV2 | 1 (25) |
| Hospitalized contactsd | ||||||||||
| High risk | 6 | 6 (100) | 4 (67) | 6 (100) | 1 (17) | 0 (0) | 1 (17): 1 HKU1 | 2 (33): 2 IAV | 0 (0) | 1 (17) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| Total | 172 | 168 (98) | 70 (40) | 73 (42)c | 1 (1) | 2 (3) | 12 (16) | 24 (33) | 6 (8) | 13 (18) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: HKU, human coronavirus HKU; IAV, influenza A virus; IBV, influenza B virus; NL63, human coronavirus NL63; PIV2, parainfluenza virus B; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aPer 100 contacts.
bPer 100 contacts sampled.
cOne or more asymptomatic contacts were tested.
dContacts of the chalet, hospitalized for isolation and systematically tested.
Number of High-risk and Low-risk Contacts and Virological Results for Respiratory Viruses, Contamines-Montjoie, France, January–February 2020
| Risk Category . | Contacts, No. . | Contacteda . | Possible Casesa . | Sampleda . | SARS-CoV-2b . | Adenovirusb . | Human Coronavirusb . | Influenzab . | Parainfluenzab . | Picornavirusb . |
|---|---|---|---|---|---|---|---|---|---|---|
| School A | ||||||||||
| High risk | 31 | 31 (100) | 18 (58) | 19 (61%)c | 0 (0) | 0 (0) | 6 (32): 2 HKU1, 4 NL63 | 6 (32): 3 IAV, 3 IBV | 3 (16): 3 PIV2 | 3 (16) |
| Low risk | 24 | 24 (100) | 23 (96) | 23 (96) | 0 (0) | 1 (4) | 2 (9): 2 HKU1 | 11 (48): 10 IAV, 1 IBV | 1 (4): 1 PIV2 | 3 (13) |
| School B | ||||||||||
| High risk | 5 | 5 (100) | 2 (40) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| School C | ||||||||||
| High risk | 25 | 25 (100) | 10 (40) | 10 (40) | 0 (0) | 0 (0) | 0 (0) | 3 (30): 1 IAV, 2 IBV | 0 (0) | 3 (30) |
| Low risk | 1 | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Ski club | ||||||||||
| High risk | 5 | 5 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Low risk | 11 | 10 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Other community contacts | ||||||||||
| High risk | 12 | 11 (92) | 8 (67) | 8 (67) | 0 (0) | 1 (13) | 2 (25): 1 HKU1, 1 NL63 | 2 (25): 2 IAV | 0 (0) | 1 (13) |
| Low risk | 52 | 51 (98) | 4 (8) | 4 (8) | 0 (0) | 0 (0) | 1 (25): 1 NL63 | 0 (0) | 2 (50): 1 PIV2 | 1 (25) |
| Hospitalized contactsd | ||||||||||
| High risk | 6 | 6 (100) | 4 (67) | 6 (100) | 1 (17) | 0 (0) | 1 (17): 1 HKU1 | 2 (33): 2 IAV | 0 (0) | 1 (17) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| Total | 172 | 168 (98) | 70 (40) | 73 (42)c | 1 (1) | 2 (3) | 12 (16) | 24 (33) | 6 (8) | 13 (18) |
| Risk Category . | Contacts, No. . | Contacteda . | Possible Casesa . | Sampleda . | SARS-CoV-2b . | Adenovirusb . | Human Coronavirusb . | Influenzab . | Parainfluenzab . | Picornavirusb . |
|---|---|---|---|---|---|---|---|---|---|---|
| School A | ||||||||||
| High risk | 31 | 31 (100) | 18 (58) | 19 (61%)c | 0 (0) | 0 (0) | 6 (32): 2 HKU1, 4 NL63 | 6 (32): 3 IAV, 3 IBV | 3 (16): 3 PIV2 | 3 (16) |
| Low risk | 24 | 24 (100) | 23 (96) | 23 (96) | 0 (0) | 1 (4) | 2 (9): 2 HKU1 | 11 (48): 10 IAV, 1 IBV | 1 (4): 1 PIV2 | 3 (13) |
| School B | ||||||||||
| High risk | 5 | 5 (100) | 2 (40) | 2 (40) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| School C | ||||||||||
| High risk | 25 | 25 (100) | 10 (40) | 10 (40) | 0 (0) | 0 (0) | 0 (0) | 3 (30): 1 IAV, 2 IBV | 0 (0) | 3 (30) |
| Low risk | 1 | 1 (100) | 1 (100) | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| Ski club | ||||||||||
| High risk | 5 | 5 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Low risk | 11 | 10 (100) | 0 (0) | 0 (0) | … | … | … | … | … | … |
| Other community contacts | ||||||||||
| High risk | 12 | 11 (92) | 8 (67) | 8 (67) | 0 (0) | 1 (13) | 2 (25): 1 HKU1, 1 NL63 | 2 (25): 2 IAV | 0 (0) | 1 (13) |
| Low risk | 52 | 51 (98) | 4 (8) | 4 (8) | 0 (0) | 0 (0) | 1 (25): 1 NL63 | 0 (0) | 2 (50): 1 PIV2 | 1 (25) |
| Hospitalized contactsd | ||||||||||
| High risk | 6 | 6 (100) | 4 (67) | 6 (100) | 1 (17) | 0 (0) | 1 (17): 1 HKU1 | 2 (33): 2 IAV | 0 (0) | 1 (17) |
| Low risk | 0 | … | … | … | … | … | … | … | … | … |
| Total | 172 | 168 (98) | 70 (40) | 73 (42)c | 1 (1) | 2 (3) | 12 (16) | 24 (33) | 6 (8) | 13 (18) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: HKU, human coronavirus HKU; IAV, influenza A virus; IBV, influenza B virus; NL63, human coronavirus NL63; PIV2, parainfluenza virus B; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aPer 100 contacts.
bPer 100 contacts sampled.
cOne or more asymptomatic contacts were tested.
dContacts of the chalet, hospitalized for isolation and systematically tested.
Except for case 13 (tertiary case), no SARS-CoV-2 virus was detected in any of the contacts monitored. However, other seasonal respiratory viruses were detected in 64% (n = 46) of the tested contacts, representing 62% and 67% of high- and low-risk contacts, respectively (Table 1). Overall, 33% of the viral infections detected were due to influenza (75% of influenza A(H1N1)pdm09 virus and 25% of influenza B virus) and 18% were due to a picornavirus (rhinovirus or enterovirus). At school C, 30% (3/10) of contacts of the pediatric case had a picornavirus infection (Table 1). In addition, “classical” human coronaviruses such as HUK1 and NL63 were detected in 16% of contacts without any cross-reactivity with SARS-CoV-2 molecular diagnostics.
DISCUSSION
In this international community cluster, all cases but 1 (tertiary case) were high-risk contacts of the index case, indicating that 1 case may have infected 11 other individuals. Transmission occurred only within the environment of the chalet, where the attack rate was very high. Studies suggested that the basic reproductive number (R0) for COVID-19 ranges between 2 and 3 [2, 12]. Although estimates and confidence intervals around those estimates may differ, the observed R0 in this cluster was at least 3 times higher, suggesting a possible “super-spreading” event. Several super-spreading events have occurred during other emerging coronavirus outbreaks, such as the outbreaks of the Middle East respiratory syndrome (MERS-CoV) in 2015 and severe acute respiratory syndrome coronavirus (SARS-CoV) in 2003 [13]. SARS-CoV and SARS-CoV-2 share the same cell receptor, human angiotensin-converting enzyme 2 (hACE2) [14]; as the expression of hACE2 is more important in the pneumocytes and lower airway epithelia, cases with pneumonia and/or bronchitis may both produce and (by cough or sneezing) disseminate more efficiently the virus [15]. Given their high propensity to spread infection, super-spreaders probably shed larger amounts of pathogen and for a longer period of time [16]. A complex combination of factors—including the host, the virus, the behavior of individuals, and the environment (proximity and air flow dynamics)—all likely play a role in the number of subsequent infections initiating from a single super-spreader [17]. A thorough study of those factors and environmental dynamics will be important to delineate their relative contribution to the phenomenon of super-spreading.
As the majority of persons present in the chalet on 7 February reported current or previous respiratory symptoms, they were all hospitalized and tested for SARS-CoV-2. Following several detailed interviews, it was confirmed that 1 case did not develop any symptoms. Anecdotal reports suggest the occurrence of asymptomatic cases of COVID-19. In recently published reports, SARS-CoV-2 was detected in asymptomatic travelers returning to Germany from China [18] and in asymptomatic family members in 2 family clusters in China [19, 20]. The possibility of transmission from an asymptomatic individual is not clear. Recently published reports have suggested potential transmission during the incubation period of the index patients [21, 22]. In line with another recently published study [23], our study indicated that an asymptomatic individual had similar viral load as a symptomatic case, both at low levels, suggesting a transmission potential of asymptomatic or minimally symptomatic cases. To confirm this finding, we used quantitative and normalization methods, to allow valid comparisons of the different specimens. As our tertiary case was exposed to 2 symptomatic and 1 asymptomatic case in apartment 1 of the chalet, it was not possible to disentangle who of those was the source of infection. However, given that the virus can remain viable and infectious on surfaces up to days, environmental contamination via the index case cannot be excluded [24]. Future seroprevalence studies will be warranted to estimate the prevalence of asymptomatic infections, and household and viral shedding studies to determine the transmission dynamics of asymptomatic individuals [25].
All symptomatic cases of this cluster treated in France initially presented with mild respiratory symptoms and none of them showed signs of severe clinical illness. This mild clinical picture contrasts with the first reports of COVID-19 cases series, which featured a high proportion of severe pneumonia and a considerable case-fatality rate [6, 7]. This indicates that the proportion of mild SARS-CoV-2 may be higher than initially suspected. In our cluster, cases could continue their activities, including skiing (and schooling for the child) while symptomatic, and they interacted with many other individuals, increasing possibilities for transmission. Despite those interactions, only 1 tertiary case was detected and linked to the chalet. It is unlikely that we missed symptomatic cases, as we monitored and tested all contacts who developed symptoms during the 14-day follow-up period. However, we may have missed asymptomatic cases.
Particularly, the infected child, despite interactions with a large number of contacts in different schools, did not transmit the disease, as evidenced by the large number of negative results of his tested contacts. However, the high proportion of picornavirus and influenza infections among his contacts at the schools indicated transmission of those viruses within those settings. Similarly, we observed that the family cluster allowed the dissemination of picornaviruses or influenza A viruses in the 3 children, while SARS-CoV-2 was detected in only 1 child. These 2 observations suggest that picornavirus and influenza infections are more easily transmitted than SARS-CoV-2. It is possible that viral interference in the host may impact the individual susceptibility to another viral respiratory infection as observed during the 2009 influenza pandemic and other winter seasons between A(H1N1) influenza virus and respiratory syncytial virus [26, 27]. It is also possible that the very low viral load of the pediatric case and the subsequent lack of transmission might be related to his coinfection and the co-circulation of respiratory viruses. Viral load was only tested 8 days after his onset of symptoms. The child continued his normal activities and interactions as his symptoms were mild. Current evidence indicates that children develop COVID-19 less often than adults and the clinical manifestations of the disease are milder [28, 29]. The above suggest that children, being less likely to be infected and more likely to develop mild disease, may play a less important role in the transmission of this novel virus.
The tertiary case has been regularly screened for SARS-CoV-2 infection through the testing of NP specimens. Following the development of fever and dry cough and abnormalities on the CT scan, 2 endotracheal aspirates collected on 2 consecutive days were positive for SARS-CoV-2. However, the NP swabs collected on the same days remained negative. Earlier reports noted a higher detection rate in lower respiratory tract specimens than in upper respiratory tract specimens [30]. This dissociation between upper and lower respiratory tract results has been recently documented and may be related with compartmentalization of infection along time and with the virus receptor distribution discussed earlier [14, 15, 30–32]. Most screening strategies rely on upper respiratory tract specimens only. This case suggests that some clinical presentations may be limited to lower respiratory tract infection, not necessarily severe. In our case, only 2 successive specimens were positive, with a low viral load. This suggests an unlikely risk of transmission, but a risk of missed diagnosis if lower respiratory tract specimens are not screened in suspected cases with signs of lower respiratory tract infection.
This cluster occurred during the winter season. As the case definition was sensitive, the proportion of possible cases in contacts with respiratory signs/symptoms was high. Of those, 64% tested positive for other respiratory viruses circulating in the community during this winter period. Due to public anxiety, many parents asked for a screening test to exclude the possibility of COVID-19 among their children. However, following the surveillance protocol, health professionals mostly tested symptomatic individuals who met the case definition of a possible case. Rapid and accurate risk communication targeting the contacts of the cases and the general public of the skiing resort were crucial for the management of this outbreak.
No further tertiary cases were detected within the 14-day follow-up period of the numerous contacts, indicating that this transmission chain was successfully interrupted. This investigation required collaboration with the health authorities of 5 different countries (France, the UK, Spain, Switzerland, and Australia) with the support of EWRS and International Health Regulations national focal points, underlying the added value of international notification systems. The subsequent generation of cases was detected within 24 hours after the initial notification and an extensive contact-tracing exercise was rolled out on the next day, requiring quick and coordinated mobilization of approximately 100 professionals during the weekend and the collaboration of multidisciplinary teams at the local and national level. Preparedness following the experience of previous emerging disease outbreaks, such as SARS, MERS-CoV, A(H1N1)pdm09, and Ebola, facilitated a quick and coordinated response [8].
Despite extensive contact tracing, some contacts were either impossible to trace (eg, people sharing ski cabins or lifts, co-travelers in public transportations who did not book their tickets online) or evaluated as negligible because of short and/or distant contacts (eg, casual contacts). Accidental events at risk of transmission in such occasions, such as an episode of cough or sneezing, cannot be ruled out.
CONCLUSIONS
The investigation of this cluster of COVID-19 in France indicated the occurrence of 1 asymptomatic infection with similar viral load as a symptomatic case, suggesting a transmission potential of asymptomatic cases. The occurrence of a case in a child, coinfected with other respiratory viruses, who did not transmit the disease despite interactions with his classmates suggests that children might not be an important source of transmission of this novel virus. The potential dissociation between upper and lower respiratory tract results underscores the need for close monitoring of the clinical evolution and screening of suspected COVID-19 cases. Finally, preparation, international collaboration, early warning and response systems, and a multidisciplinary approach are essential elements for the effective management of and quick response to emerging infectious diseases.
Notes
Investigation Team. Elise Brottet, Delphine Casamatta, Yves Gallien, Scarlett George, Delphine Viriot, Fatima Ait Belghiti, Sibylle Bernard-Stoecklin, Jean-Claude Desenclos, Coralie Giese, Didier Ghislain, Magali Gounon, Nathalie Grangeret, Cécile Marie, Bruno Morel, Muriel Deher, Anne-Sophie Ronnaux Baron, Geneviève Courbis, Nathalie Ragozin, Monika Wolska, Eric Serange, Delphine Mercatello, Soraya Aiouaz, Martine Valette, Emilie Frobert, Laurence Josset, Vanessa Escuret, Florence Morfin, Geneviève Billaud, Myriam Blanc, Julie Arata-Bardet, Marie Froidure, Marion Le Maréchal, Patricia Pavese, Isabelle Pierre, Agathe Becker, Pierre Chauvelot, Anne Conrad, Tristan Ferry, Patrick Miailhes, Thomas Perpoint, Cécile Pouderoux, Sandrine Roux, Florent Valour, Marie-France Lutz, Anne Pouvaret, Virginie Vitrat, Mylène Maillet, Cécile Janssen, Emilie Piet, Alexie Bosch, Anne-Laure Destrem, Margaux Isnard, Thibault Challan-Belval, Chloe Wackenheim, Alice Couturier, Gael Gheno, Thierry Roupioz, Nicolas Lucet, Stéphane Ayouni, Mireille Vincent, Servicio de Epidemiología, Dirección General de Salud Pública del Gover Balear, Virginie Masserey Spicher, Catherine Bourquin, Jeanine Stoll, Pascal Chaud, Anne-Laure Mounayar.
Author contributions. K. D., O. E., T. B., and A. G. wrote the first draft of the manuscript. K. D., T. B., S. C., G. S., A. M., Z. B., J. B., S. V., E. F., A. T., R. V., D. L., B. C., and C. S. were involved in the epidemiological investigation and contact-tracing activities. A. G., B. D. M., P. B., and B. L. did the microbiological analysis. O. E., E. B. N., F. A., C. L., E. F., V. T., F. B., and C. C. were involved in the clinical management of the cases, the evaluation of the risk of the contacts, and the analysis and the interpretation of clinical data. All authors were responsible for the interpretation of the findings and the writing of the report. All authors revised and approved the manuscript.
Acknowledgments. The authors thank all of the patients and all the multiple actors who contributed to this investigation. As this was a response to an urgent public health event, an ethics committee approval was not needed.
Disclaimer. All authors are employees of publicly funded institutes.
Financial support. All authors are employees of publicly funded institutes. There was no external funding for this work.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
Author notes
K. D., O. E., T. B., A. G., C. C., B. L., B. C., and C. S. contributed equally to this work.
The investigation team is listed in the Notes.





Comments