Water Quality and Macroinvertebrate Community in Dryland Streams: The Case of the Tehuacán-Cuicatlán Biosphere Reserve (México) Facing Climate Change
Abstract
:1. Introduction
2. Materials and Methods
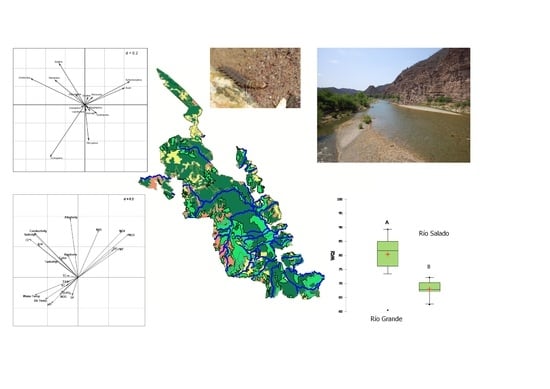
2.1. Study Area
2.2. Water and Macroinvertebrate Sampling
2.3. Water Quality Analysis and Identification of Aquatic Macroinvertebrates
2.4. Land Use and Climate
2.5. Data Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Streams
3.2. WQI
3.3. Land Use and Climate
3.4. Macroinvertebrates
3.4.1. Family Richness
3.4.2. Biological Indices
3.5. Relationship between Macroinvertebrate Assemblages and Physicochemical Variables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bastin, J.-F.; Berrahmouni, N.; Grainger, A.; Maniatis, D. The extent of forest in dryland biomes. Science 2017, 356, 635–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Coservation: The Critical Role of Hotspots. In Biodiversity Hotspots; Zachos, F., Habel, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. Available online: https://doi.org/10.1007/978-3-642-20992-5_1 (accessed on 3 May 2019).
- Davies, B.R.; Thoms, M.C.; Walker, K.F.; O’Keefe, J.H.; Gore, J.A. Drylands Rivers: Their ecology, conservation and management. In The Rivers Handbook: Hydrological and Ecological Principles; Calow, P., Petts, G.E., Eds.; Blackwell: Oxford, UK, 1994; Volume 2, pp. 484–512. Available online: https://doi.org/10.1002/9781444313871.ch25 (accessed on 3 May 2019).
- Walker, K.F.; Sheldon, F.; Puckridge, J.T. A perspective on dryland river ecosystems. River Res. Appl. 1995, 11, 85–104. [Google Scholar] [CrossRef]
- Powell, D.M. Drylands Rivers: Processes and Forms. In Geomorphology of Desert Environments, 2nd ed.; Parsons, A.J., Abrahams, A.D., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 333–372. Available online: https://doi.org/10.1007/978-1-4020-5719-9_12 (accessed on 3 May 2019).
- Amoros, C.; Bornotte, G. Connectivity and biocomplexity in waterbodies of riverine floodplains. Freshw. Biol. 2002, 47, 761–776. [Google Scholar] [CrossRef]
- Poff, N.L.; Olden, J.D.; Merritt, D.M.; Pepin, D.M. Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. USA 2007, 104, 5732–5737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldon, F. Incorporating natural variability into the assessment of ecological health in Australian Dryland rivers. Hydrobiologia 2005, 552, 45–56. [Google Scholar] [CrossRef]
- Few, R.; Satyal, P.; McGahey, D.M.; Leavy, J.; Budds, J.; Assen, M.; Camfield, L.; Louser, D.; Adnew, M.; Bewket, W. Vulnerability and Adaptation to Climate Change in Semi-Arid Areas in East Africa. ASSAR Working Paper, ASSAR PMU 2015. Available online: http://www.assar.uct.ac.za/sites/default/files/image_tool/images/138/RDS_reports/EAST-AFRICA/East%20Africa%20RDS%20full%20report%20-%20revised.pdf (accessed on 3 May 2019).
- Arab Water Council. Vulnerability of arid and semi-arid regions to climate change—Impacts and adaptive strategies. In Perspectives on Water and Climate Change Adaptation; Arab Water Council: Cairo, Egypt, 2019; Available online: https://www.preventionweb.net/files/12914_PersPap09.AridandSemiAridRegions1.pdfñAqW23 (accessed on 3 May 2019).
- Mott Lacroix, K.E.; Tapia, E.; Springer, A. Environmental flows in the desert rivers of the United States and Mexico: Synthesis of available data and gap analysis. J. Arid Environ. 2017, 140, 67–78. [Google Scholar] [CrossRef]
- SAGARPA. Available online: http://www.atlaszonasaridas.com.mx/ (accessed on 1 May 2019).
- SEMARNAT. Available online: https://www.gob.mx/semarnat/prensa/el-area-natural-protegida-tehuacan-cuicatlan-ya-es-patrimonio-de-la-humanidad-163342?idiom=es (accessed on 1 May 2019).
- UNESCO. Available online: http://www.unesco.org/new/es/media-services/single-view/news/tehuacan_cuicatlan_a_la_lista_del_patrimonio_mundial_como/ (accessed on 2 May 2019).
- Téllez-Valdés, O.; Dávila-Aranda, P. Áreas protegidas y cambio climático: Un estudio de caso de los cactos en la Reserva de la Biosfera Tehuacán-Cuicatlán, México. Conserv. Biol. 2003, 17, 846–853. [Google Scholar]
- Blanchette, M.L.; Pearson, R.G. Macroinvertebrate assemblages in rivers of the Australian dry tropics are highly variable. Freshw. Sci. 2012, 31, 865–881. [Google Scholar] [CrossRef]
- Chessman, B.C.; Thurtell, L.A.; Royal, M.J. Bioassessment in A Harsh Environment: A comparison of macroinvertebrate assemblages at reference and assessment sites in an Australian inland river system. Environ. Monit. Assess. 2006, 119, 303–330. [Google Scholar] [CrossRef]
- Kaboré, I.; Moog, O.; Alp, M.; Guenda, W.; Koblinger, T.; Mano, K.; Ouéda, A.; Ouédraogo, R.; Trauner, D.; Melcher, A.H. Using macroinvertebrates for ecosystem health assessment in semi-arid streams of Burkina Faso. Hydribiologia 2015, 766, 57–74. [Google Scholar] [CrossRef]
- Mabidi, A.; Bird, M.S.; Perissinotto, R. Distribution and diversity of aquatic macroinvertebrate assemblages in a semi-arid region earmarket for shale gas exploration (Eastern Cape Karoo, South Africa). PLoS ONE 2017, 12, e0178559. [Google Scholar] [CrossRef] [PubMed]
- Mellado, A.; Suárez, M.L.; Vidal-Abarca, M.R. Biological traits of stream macroinvertebrates from a semiarid catchment: Patterns along complex environmental gradients. Freshw. Biol. 2008, 53, 1–21. [Google Scholar]
- Rocha, L.G.; Medeiros, E.S.F.; Andrade, H.T.A. Influence of flow variability on macroinvertebrate assemblages in an intermittent stream of semi-arid Brazil. J. Arid Environ. 2012, 85, 33–40. [Google Scholar] [CrossRef]
- Giam, X.; Chen, W.; Scriever, T.A.M.; van Driesche, R.; Muneepeerakul, R.; Lytle, D.A.; Olden, J.D. Hydrology drives seasonal variation in dryland stream macroinvertebrate communities. Aquat. Sci. 2016, 79, 705–717. [Google Scholar] [CrossRef]
- Moreno, J.L.; Angeler, D.G.; de las Heras, J. Seasonal dynamics of macroinvertebrate communities in a semiarid saline springs stream with contrasting environmental conditions. Aquat. Ecol. 2010, 44, 177–193. [Google Scholar] [CrossRef]
- Alvial, I.E.; Tapia, D.H.; Castro, M.J.; Duran, B.C.; Verdugo, C.A. Analysis of benthic macroinvertebrates and biotic indices to evaluate water quality in rivers impacted by mining activities in northern Chile. Knowl. Manag. Aquat. Ecosyst. 2012, 407, 1–16. [Google Scholar] [CrossRef]
- Miserendino, M. Macroinvertebrate functional organization and water quality in a large arid river from Patagonia (Argentina). Int. J. Limnol. 2007, 43, 133–145. [Google Scholar] [CrossRef]
- Ruiz-Picos, R.A.; Sedeño-Díaz, J.E.; López-López, E. Assessing ecological impairments in Neotropical rivers of Mexico: Calibration and validation of the Biomonitoring Working Party Index. Int. J. Environ. Sci. Technol. 2017, 4, 1835–1852. [Google Scholar] [CrossRef]
- SEMARNAT; CONANP. Programa de Manejo de la Reserva de la Biosfera Tehuacán-Cuicatlán, 1st ed.; SEMARNAT: Ciudad de México, Mexico, 2013.
- Brunet, J. Geologic studies. In The Prehistory of the Tehuacan Valley; Environment and Subsistance; Byers, D.S., Ed.; Robert S. Peabody Foundation: Austin, TX, USA, 1967; Volume I, pp. 66–90. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática (Para Adaptarlo a las Condiciones de la República Mexicana, 6th ed.; UNAM: Mexico City, Mexico, 2004; p. 90. [Google Scholar]
- INEGI. Available online: http://www.beta.inegi.org.mx/temas/mapas/climatologia/ (accessed on 1 May 2019).
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton Benthic Macroinvertebrates and Fish; US Environmental Protection Agency: Washington, DC, USA, 1999.
- Thorp, J.H.; Covich, A.P. An Overview of Freshwater Habitats. In Ecology and Classification of North American Freshwater Invertebrates, 2nd ed.; Academic Press: Fort Collins, CO, USA, 2001. [Google Scholar]
- Merrit, R.W.; Cummins, K.W.; Berg, M.B. An Introduction to the Aquatic Insects of North America; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 2008. [Google Scholar]
- Rodríguez-Romero, A.J.; Rico-Sánchez, A.E.; Mendoza-Martínez, E.; Gómez-Ruiz, A.; Sedeño-Díaz, J.E.; López-López, E. Impact of Changes of Land Use on Water Quality, from Tropical Forest to anthropogenic Occupation: A Multivariate Approach. Water 2018, 10, 1518. [Google Scholar] [CrossRef]
- Dinius, S.H. Desing of an index of water quality. Water Resour. Bull. 1987, 23, 833–843. [Google Scholar] [CrossRef]
- Sing, K.P.; Malik, A.; Mohan, D.; Sinha, S. Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): A case study. Water Res. 2004, 38, 3980–3992. [Google Scholar] [CrossRef] [PubMed]
- Massart, D.L.; Vander, H.Y. Practical data handling: From tables to visuals, principal component analysis, Part 1. LG GC Eur. 2004, 17, 586–591. [Google Scholar]
- Li, X.; Li, P.; Wang, D.; Wang, Y. Assessment of temporal and spatial variations in waterquality using multivariate statistical methods: A case study ofthe Xin’anjiang River, China. Front. Environ. Sci. Eng. 2014, 8, 895–904. [Google Scholar] [CrossRef]
- Evansa, C.D.; Daviesa, T.D.; Wigington, P.J., Jr.; Tranter, M.; Kretser, W.A. Use of factor analysis to investigate processes controlling the chemical composition of four streams in the Adirondack Mountains, New York. J. Hydrol. 1996, 185, 297–316. [Google Scholar] [CrossRef]
- Grande, J.A.; González, A.; Beltrán, R. Application of factor analysis to the study of contamination in the aquifer system of Ayamonte-Huelva (Spain). Groundwater 1996, 34, 155–161. [Google Scholar] [CrossRef]
- Razmkhah, H.; Abrishamchi, A.; Torkian, A. Evaluation of spatial and temporal variation in water quality by pattern recognition techniques: A case study on Jajrood River (Tehran, Iran). J. Environ. Manag. 2010, 91, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Alberto, W.D.; Del Pilar, D.M.; Valeria, A.M.; Fabiana, P.S.; Cecilia, H.A.; De Los Angeles, B.M. Pattern recognition techniques for the evaluation of spatial and temporal variations in wáter quality. A case study: Suquía River basin. (Córdoba-Argentina). Water Res. 2001, 35, 2881–2894. [Google Scholar]
- Dolédec, S.; Chessel, D. Co-inertia analysis: An alternative method for studying species-environment relationships. Freshw. Biol. 1994, 31, 277–294. [Google Scholar] [CrossRef]
- Franquet, E.; Chessel, S. Approche statistique des composantes spatiales et temporelles de la relation faune-milieu. C. R. Acad. Sci. Paris Sci. Vie. 1994, 317, 202–206. [Google Scholar]
- Franquet, E.; Dolédec, S.; Chessel, D. Using multivariate analyses for separating spatial and temporal effects within species-environment relationships. Hydrobiologia 1995, 300–301, 97–112. [Google Scholar]
- Chessel, D.; Dolédec, S.; ADE softwere. Multivariate Analysis and Graphical Display for Environmental Data Version 3.4; Volume 1 User Manual, Volume 2 Examples, Volume 3 Graphics Documentation; Univeristy Lyon: Lyon, France, 1992; p. 523. [Google Scholar]
- Thioulouse, J.; Chessel, D.; Dolédec, S.; Olivier, J.M. ADE-4: A multivariate analysis and graphical display software. Stat. Comput. 1997, 7, 75–83. [Google Scholar] [CrossRef]
- van der Aa, M. Classification of mineral water types and comparison with drinking water standards. Environ. Geol. 2003, 44, 554–563. [Google Scholar] [CrossRef] [Green Version]
- Rhoades, J.D.; Kandiah, A.; Mashali, A.M. The Use of Saline Waters for Crop Production—FAO Irrigation and Drainage Paper 48; Food and Agriculture Organization of the United Nations: Rome, Italy, 1992. [Google Scholar]
- SGS-U.S. Geological Survey Office of Water Quality. USGS Water-Quality Information: Water Hardness and Alkalinity. Available online: usgs.gov.water.usgs.gov/owq/hardness-alkalinity.html (accessed on 3 April 2019).
- Kerr, J. Multiple land use activities drive riverine salinization in a large, semi-arid river basin in western Canada. Limnol. Oceanogr. 2017, 62, 1331–1345. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Available online: https://www.inegi.org.mx/temas/edafologia/ (accessed on 3 May 2019).
- Stum, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Crosa, G.; Froebrich, J.; Nikolayenki, V.; Stegani, F.; Galli, P.; Calamari, D. Spatial and seasonal variations in the water quality of the Amu Darya River (Central Asia). Water Res. 2006, 40, 2237–2245. [Google Scholar] [CrossRef]
- Ahearn, D.S.; Sheibley, R.W.; Dahlgren, R.A.; Anderson, M.; Johnson, J.; Tate, K.W. Land use and land cover influence on water quality in the last free-flowing river draining the western Sierra Nevada, California. J. Hydrol. 2005, 313, 234–247. [Google Scholar] [CrossRef]
- Lawniczak, A.E.; Zbierska, J.; Nowak, B.; Achtenberg, K.; Grzeskowiak, A.; Kanas, K. Impact of agriculture and land use on nitrate contamination in groundwater and running waters in central-west Poland. Environ. Monit. Assess. 2016, 188, 172. [Google Scholar] [CrossRef]
- Wen, Y.; Schoups, G.; van de Giesen, N. Organic pollution of rivers: Combined threats of urbanization, livestock farming and global climate change. Sci. Rep. 2017, 7, 43289. [Google Scholar] [CrossRef]
- Uriarte, M.; Yackulic, C.B.; Lim, Y.; Arce-Nazario, J.A. Influence of land use on water quality in a tropical landscape: A multi-scale analysis. Landsc. Ecol. 2011, 26, 1151–1164. [Google Scholar] [CrossRef]
- Sedeño-Díaz, J.E.; López López, E. Water Quality in the Río Lerma, Mexico: An Overviewof the Last Quarter of the Twentieth Century. Water Resour. Manag. 2007, 21, 1797–1812. [Google Scholar] [CrossRef]
- Allan, D. Landscapes and rivescapes: The influence of land use on stream scosystems. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 57–84. [Google Scholar] [CrossRef]
- Arheimer, B.; Lidén, R. Nitrogen and phosphorus concentrations from agricultural catchments-influence of spatial and temporal variables. J. Hydrol. 2000, 227, 140–159. [Google Scholar] [CrossRef]
- Wise, D.R.; Johnson, H.M. Suface-water nutrient conditions and sources in the United States Pacific Northwest. J. Am. Water Resour. Assoc. 2011, 47, 1110–1135. [Google Scholar] [CrossRef] [PubMed]
- Borrok, D.M.; Engle, M.A. The role of climate in increasing salt loads in Dryland Rivers. J. Arid Environ. 2014, 111, 7–13. [Google Scholar] [CrossRef]
- CIIDIR. Proyecto: Vulnerabilidad y Adaptación de Los Sectores y Sistemas de Interés Para el Estado de Oaxaca, Ante Los Efectos del Cambio Climático; Instituto Politécnico Nacional Oaxaca: Oaxaca, Mexico, 2013. [Google Scholar]
- Cañedo-Argüelles, M.; Boga, M.T.; Lytle, D.A.; Prat, N. Are Chironomidae (Diptera) good indicators of water scarcity? Dyland streams as a case study. Ecol. Indic. 2016, 71, 155–162. [Google Scholar] [CrossRef]
- Bunn, S.E.; Davies, P.M. Community structure of the macroinvertebrates fauna and water quality of a saline river system in south-western Australia. Hydrobiologia 1992, 248, 143–160. [Google Scholar] [CrossRef]
- Zinchenko, T.D.; Golovatyuk, L.V. Salinity tolerance of macroinvertebrates in stream waters. Arid Ecosyst. 2013, 3, 113–121. [Google Scholar] [CrossRef]
- Timpano, A.J.; Schoenholtz, S.H.; Soucek, D.J.; Zipper, C.E. Benthic macroinvertebrate community response to salinization in headwater streams in Appalachia USA over multiple years. Ecol. Indic. 2018, 91, 645–656. [Google Scholar] [CrossRef]
- Alhejoj, I.; Salameh, E.; Bandel, K. Mayflies (Order Ephemeroptera): An effective indicator of water bodies conditions in Jordan. IJSRES 2014, 2, 361–370. [Google Scholar] [CrossRef]
- Kefford, B.J.; Dalton, A.; Palmer, C.S.; Nugegoda, D. The salinity tolerance of eggs and hathlings of selected aquatic macroinvertebrates in south-east Australia and South Africa. Hydrobiologia 2004, 517, 179–192. [Google Scholar] [CrossRef]
- Kefford, B.J.; Fields, E.J.; Clay, C.; Nugegoda, D. Salinity tolerance of riverine microinvertebrates from the southern Murray-Darling Basin. Mar. Freshw. Res. 2007, 58, 1019–1031. [Google Scholar] [CrossRef]
- Jaeger, K.L.; Olden, J.D.; Pelland, N.A. Climate change poised to threaten hydrologic connectivity and endemic fishes in dryland streams. Proc. Natl. Acad. Sci. USA 2014, 111, 13894–13899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vammen, K.; Vaux, H. Water Quality in the Americas Risks and Opportunities; Interamerican Network of Academies of Sciences: Ciudad de México, Mexico, 2019; pp. 12–18. [Google Scholar]
- Schlaepfer, D.R.; Bradford, J.B.; Lauenroth, W.K.; Munson, S.M.; Tietjen, B.; Hall, S.A.; Wilson, S.D.; Duniway, M.C.; Jia, G.; Pyke, D.A.; et al. Climate change reduces extent of temperature drylands and intensifies drought in deep soil. Nat. Commun. 2017, 8, 14196. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cánovas, C.; Sánchez-Fernández, D.; Cañedo-Argüelles, M.; Millán, A.; Velasco, J.; Acosta, R.; Fortuño, P.; Otero, N.; Soler, A.; Bonada, N. Do all roads to Rome? Exploring community trajectories in response to anthropogenic salinization and dilution of rivers. Philos. Trans. R. Soc. B 2018, 374, 20180009. [Google Scholar] [CrossRef]










| Río Salado | Río Grande | |
|---|---|---|
| Alkalinity (mg L−1) a | 261.89 (±24.6) | 161.21 (±7.90) |
| Cl- (mg L−1) a | 379.60 (±55.29) | 28.45 (±13.86) |
| Col. Fec. (MPN) | 281.68 (±171.09) | 245.85 (±69.43) |
| Col. Total (MPN) | 349.26 (±167.96) | 393.64 (±89.59) |
| Color (U Pt-Co) | 65.4 (±38.66) | 12.12 (±2.04) |
| Cond µS/cm a | 2627.6 (±33.5) | 403.82 (±23.56) |
| BOD5 (mg L−1) | 1.40 (±0.28) | 1.520 (±0.18) |
| Hardness (mg L−1) | 156.55 (±28.26) | 103.90 (±6.96) |
| NH3 (mg L−1) | 0.28 (±0.11) | 0.16 (±0.03) |
| NO2 (mg L−1) | 0.01 (±0.001) | 0.01 (±0.001) |
| NO3 (mg L−1) | 1.76 (±0.30) | 1.78 (±0.16) |
| NT (mg L−1) | 2.71 (±0.50) | 2.46 (±0.34) |
| DO (mg L−1) | 8.59 (±0.51) | 8.91 (±0.20) |
| O-PO4 (mg L−1) | 0.52 (±0.15) | 0.47 (±0.08) |
| pH | 8.90 (±0.29) | 9.00 (±0.15) |
| PT (mg L−1) | 0.76 (±0.14) | 1.02 (±0.12) |
| Salinity (PSU) a | 1.35 (±0.18) | 0.18 (±0.01) |
| SO4 (mg L−1) a | 283.15 (±37.05) | 33.25 (±6.20) |
| TSS (mg L−1) | 66.9 (±48.88) | 16.01 (±6.15) |
| Temp. water (°C) b | 25.36 (±1.22) | 22.46 (±0.50) |
| Temp. Air (°C) b | 27.38 (±1.48) | 25.06 (±0.65) |
| Turbidity (UFT) | 84.43 (±28.54) | 56.54 (±4.76) |
| Current velocity (m/s) | 1.27 (±0.39) | 1.19 (±0.27) |
| Sub-Basin | Study Site | EPT | Oligo-Chiro | Study Site | EPT | Oligo-Chiro | Study Site | EPT | Oligo-Chiro |
|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | |||||||
| Río Grande | CH Ap | 64.81 | 7.41 | CH Ap | 71.17 | 0.36 | CH Jan | 45.13 | 2.73 |
| QT Ap | 51.67 | 7.84 | QT Ap | 47.24 | 0.06 | QT Jan | 39.39 | 3.08 | |
| RG Ap | 37.50 | 3.19 | RG Ap | 45.48 | 0.23 | RG Jan | 40.33 | 0.58 | |
| SNP Ap | 75.48 | 0.05 | SNP Ap | 30.95 | 1.30 | SPN Jan | 82.07 | 0.01 | |
| SMIp Ap | 95.24 | 0.00 | SMIp Ap | 5.66 | 3.19 | SMIp Jan | 61.58 | 0.09 | |
| SMIs Ap | 34.03 | 0.00 | SMIs Ap | 41.10 | 0.01 | SMIS Jan | 41.22 | 0.14 | |
| TX Ap | 88.46 | 0.04 | SD Ap | 60.56 | 1.59 | SD Jan | 54.22 | 0.05 | |
| SD Ap | 61.81 | 0.16 | TV Ap | 59.74 | 3.95 | VT Jan | 77.76 | 1.09 | |
| TV Ap | 72.56 | 0.04 | VT Ap | 41.89 | 0.05 | ||||
| CH Sep | 46.15 | 24.62 | CH Sep | 25.80 | 0.61 | ||||
| QT Sep | 21.54 | 1.72 | QT Sep | 43.79 | 4.97 | ||||
| RG Sep | 60.00 | 32.00 | RG Sep | 45.72 | 4.76 | ||||
| SNP Sep | 58.50 | 0.54 | SNP Sep | 59.29 | 2.17 | ||||
| SMIp Sep | 5.19 | 0.00 | SMIp Sep | 53.21 | 0.03 | ||||
| SMI Sep | 73.81 | 0.00 | SMIs Sep | 89.23 | 0.25 | ||||
| TX Sep | 87.68 | 1.45 | SD Sep | 62.65 | 1.55 | ||||
| SD Sep | 68.53 | 0.02 | TV Sep | 49.12 | 0.00 | ||||
| TV Sep | 35.29 | 0.00 | VT Sep | 57.47 | 3.37 | ||||
| Río Salado | BO Ap | 27.59 | 0.70 | BO Ap | 22.94 | 0.06 | BO Jan | 26.67 | 15.76 |
| RS Ap | 75.00 | 1.34 | RS Ap | 74.58 | 1.40 | RS Jan | 76.15 | 0.94 | |
| BO Sep | 17.39 | 13.04 | BO Sep | 14.66 | 0.07 | ||||
| RS Sep | 0.00 | 0.00 | RS Sep | 23.53 | 35.35 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, E.; Sedeño-Díaz, J.E.; Mendoza-Martínez, E.; Gómez-Ruiz, A.; Martínez Ramírez, E. Water Quality and Macroinvertebrate Community in Dryland Streams: The Case of the Tehuacán-Cuicatlán Biosphere Reserve (México) Facing Climate Change. Water 2019, 11, 1376. https://doi.org/10.3390/w11071376
López-López E, Sedeño-Díaz JE, Mendoza-Martínez E, Gómez-Ruiz A, Martínez Ramírez E. Water Quality and Macroinvertebrate Community in Dryland Streams: The Case of the Tehuacán-Cuicatlán Biosphere Reserve (México) Facing Climate Change. Water. 2019; 11(7):1376. https://doi.org/10.3390/w11071376
Chicago/Turabian StyleLópez-López, Eugenia, Jacinto Elías Sedeño-Díaz, Erick Mendoza-Martínez, Andrea Gómez-Ruiz, and Emilio Martínez Ramírez. 2019. "Water Quality and Macroinvertebrate Community in Dryland Streams: The Case of the Tehuacán-Cuicatlán Biosphere Reserve (México) Facing Climate Change" Water 11, no. 7: 1376. https://doi.org/10.3390/w11071376